Soluble CEACAM8 interacts with CEACAM1 inhibiting TLR2-triggered immune responses
- PMID: 24743304
- PMCID: PMC3990526
- DOI: 10.1371/journal.pone.0094106
Soluble CEACAM8 interacts with CEACAM1 inhibiting TLR2-triggered immune responses
Abstract
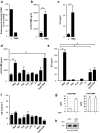
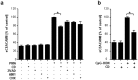
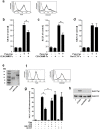
Lower respiratory tract bacterial infections are characterized by neutrophilic inflammation in the airways. The carcinoembryonic antigen-related cell adhesion molecule (CEACAM) 8 is expressed in and released by human granulocytes. Our study demonstrates that human granulocytes release CEACAM8 in response to bacterial DNA in a TLR9-dependent manner. Individuals with a high percentage of bronchial lavage fluid (BALF) granulocytes were more likely to have detectable levels of released CEACAM8 in the BALF than those with a normal granulocyte count. Soluble, recombinant CEACAM8-Fc binds to CEACAM1 expressed on human airway epithelium. Application of CEACAM8-Fc to CEACAM1-positive human pulmonary epithelial cells resulted in reduced TLR2-dependent inflammatory responses. These inhibitory effects were accompanied by tyrosine phosphorylation of the immunoreceptor tyrosine-based inhibitory motif (ITIM) of CEACAM1 and by recruitment of the phosphatase SHP-1, which could negatively regulate Toll-like receptor 2-dependent activation of the phosphatidylinositol 3-OH kinase-Akt kinase pathway. Our results suggest a new mechanism by which granulocytes reduce pro-inflammatory immune responses in human airways via secretion of CEACAM8 in neutrophil-driven bacterial infections.
Conflict of interest statement
Figures


Similar articles
-
The role of CEACAMs in neutrophil function.Eur J Clin Invest. 2024 Dec;54 Suppl 2(Suppl 2):e14349. doi: 10.1111/eci.14349. Eur J Clin Invest. 2024. PMID: 39674879 Free PMC article. Review.
-
CEACAM1 inhibits Toll-like receptor 2-triggered antibacterial responses of human pulmonary epithelial cells.Nat Immunol. 2008 Nov;9(11):1270-8. doi: 10.1038/ni.1661. Epub 2008 Oct 5. Nat Immunol. 2008. PMID: 18836450
-
Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes.J Immunol. 2002 May 15;168(10):5139-46. doi: 10.4049/jimmunol.168.10.5139. J Immunol. 2002. PMID: 11994468
-
Binding of Candida albicans to Human CEACAM1 and CEACAM6 Modulates the Inflammatory Response of Intestinal Epithelial Cells.mBio. 2017 Mar 14;8(2):e02142-16. doi: 10.1128/mBio.02142-16. mBio. 2017. PMID: 28292985 Free PMC article.
-
[Human carcinoembryonic antigen family proteins, structure and function].Postepy Hig Med Dosw (Online). 2012 Jul 20;66:521-33. doi: 10.5604/17322693.1004113. Postepy Hig Med Dosw (Online). 2012. PMID: 22922152 Review. Polish.
Cited by
-
Fusobacterium nucleatum CbpF Mediates Inhibition of T Cell Function Through CEACAM1 Activation.Front Cell Infect Microbiol. 2021 Jul 15;11:692544. doi: 10.3389/fcimb.2021.692544. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34336716 Free PMC article.
-
The role of CEACAMs in neutrophil function.Eur J Clin Invest. 2024 Dec;54 Suppl 2(Suppl 2):e14349. doi: 10.1111/eci.14349. Eur J Clin Invest. 2024. PMID: 39674879 Free PMC article. Review.
-
Proteomic Bioprofiles and Mechanistic Pathways of Progression to Heart Failure.Circ Heart Fail. 2019 May;12(5):e005897. doi: 10.1161/CIRCHEARTFAILURE.118.005897. Circ Heart Fail. 2019. PMID: 31104495 Free PMC article.
-
Moraxella catarrhalis induces CEACAM3-Syk-CARD9-dependent activation of human granulocytes.Cell Microbiol. 2016 Nov;18(11):1570-1582. doi: 10.1111/cmi.12597. Epub 2016 May 3. Cell Microbiol. 2016. PMID: 27038042 Free PMC article.
-
Immunoreceptors on neutrophils.Semin Immunol. 2016 Apr;28(2):94-108. doi: 10.1016/j.smim.2016.02.004. Epub 2016 Mar 11. Semin Immunol. 2016. PMID: 26976825 Free PMC article. Review.
References
-
- Mantovani A, Cassatella MA, Costantini C, Jaillon S (2011) Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol 11: 519–531. - PubMed
-
- Schmidt T, Zundorf J, Gruger T, Brandenburg K, Reiners AL, et al. (2012) CD66b overexpression and homotypic aggregation of human peripheral blood neutrophils after activation by a gram-positive stimulus. J Leukoc Biol 91: 791–802. - PubMed
-
- Singer BB, Scheffrahn I, Heymann R, Sigmundsson K, Kammerer R, et al. (2002) Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J Immunol 168: 5139–5146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

