Uncoupling of glomerular IgA deposition and disease progression in alymphoplasia mice with IgA nephropathy
- PMID: 24743510
- PMCID: PMC3990643
- DOI: 10.1371/journal.pone.0095365
Uncoupling of glomerular IgA deposition and disease progression in alymphoplasia mice with IgA nephropathy
Abstract
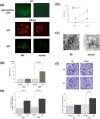
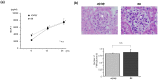
Previous clinical and experimental studies have indicated that cells responsible for IgA nephropathy (IgAN), at least in part, are localized in bone marrow (BM). Indeed, we have demonstrated that murine IgAN can be experimentally reconstituted by bone marrow transplantation (BMT) from IgAN prone mice in not only normal mice, but also in alymphoplasia mice (aly/aly) independent of IgA+ cells homing to mucosa or secondary lymphoid tissues. The objective of the present study was to further assess whether secondary lymph nodes (LN) contribute to the progression of this disease. BM cells from the several lines of IgAN prone mice were transplanted into aly/aly and wild-type mice (B6). Although the transplanted aly/aly showed the same degree of mesangial IgA and IgG deposition and the same serum elevation levels of IgA and IgA-IgG immune-complexes (IC) as B6, even in extent, the progression of glomerular injury was observed only in B6. This uncoupling in aly/aly was associated with a lack of CD4+ T cells and macrophage infiltration, although phlogogenic capacity to nephritogenic IC of renal resident cells was identical between both recipients. It is suggested that secondary LN may be required for the full progression of IgAN after nephritogenic IgA and IgA/IgG IC deposition.
Conflict of interest statement
Figures

References
-
- Burger J, Hinglais N (1968) Les Depots intercapillaries d’IgA-IgG. Urol Nephrol Paris 74: 694–695. - PubMed
-
- Tomino Y, Endoh M, Nomoto Y, Sakai H (1981) Immunoglobulin A1 and IgA nephropathy. N Engl J Med. 305: 1159–1160. - PubMed
-
- Barratt J, Feehally J (2005) IgA nephropathy. J Am Soc Nephrol 16: 2088–2097. - PubMed
-
- van den Wall Bake AW, Daha MR, Evers-Schouten J, van Es LA (1988) Serum IgA and the production of IgA by peripheral blood and bone marrow lymphocytes in patients with primary IgA nephropathy: evidence for the bone marrow as the source of mesangial IgA. Am J Kidney Dis 12: 410–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

