Curcumin significantly enhances dual PI3K/Akt and mTOR inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma Caki cells through down-regulation of p53-dependent Bcl-2 expression and inhibition of Mcl-1 protein stability
- PMID: 24743574
- PMCID: PMC3990719
- DOI: 10.1371/journal.pone.0095588
Curcumin significantly enhances dual PI3K/Akt and mTOR inhibitor NVP-BEZ235-induced apoptosis in human renal carcinoma Caki cells through down-regulation of p53-dependent Bcl-2 expression and inhibition of Mcl-1 protein stability
Erratum in
-
Correction: Curcumin Significantly Enhances Dual PI3K/Akt and mTOR Inhibitor NVP-BEZ235-Induced Apoptosis in Human Renal Carcinoma Caki Cells through Down-Regulation of p53-Dependent Bcl-2 Expression and Inhibition of Mcl-1 Protein Stability.PLoS One. 2016 Mar 14;11(3):e0151886. doi: 10.1371/journal.pone.0151886. eCollection 2016. PLoS One. 2016. PMID: 26974552 Free PMC article. No abstract available.
Abstract
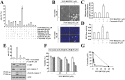
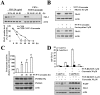
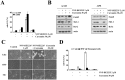
The PI3K/Akt and mTOR signaling pathways are important for cell survival and growth, and they are highly activated in cancer cells compared with normal cells. Therefore, these signaling pathways are targets for inducing cancer cell death. The dual PI3K/Akt and mTOR inhibitor NVP-BEZ235 completely inhibited both signaling pathways. However, NVP-BEZ235 had no effect on cell death in human renal carcinoma Caki cells. We tested whether combined treatment with natural compounds and NVP-BEZ235 could induce cell death. Among several chemopreventive agents, curcumin, a natural biologically active compound that is extracted from the rhizomes of Curcuma species, markedly induced apoptosis in NVP-BEZ235-treated cells. Co-treatment with curcumin and NVP-BEZ235 led to the down-regulation of Mcl-1 protein expression but not mRNA expression. Ectopic expression of Mcl-1 completely inhibited curcumin plus NVP-NEZ235-induced apoptosis. Furthermore, the down-regulation of Bcl-2 was involved in curcumin plus NVP-BEZ235-induced apoptosis. Curcumin or NVP-BEZ235 alone did not change Bcl-2 mRNA or protein expression, but co-treatment reduced Bcl-2 mRNA and protein expression. Combined treatment with NVP-BEZ235 and curcumin reduced Bcl-2 expression in wild-type p53 HCT116 human colon carcinoma cells but not p53-null HCT116 cells. Moreover, Bcl-2 expression was completely reversed by treatment with pifithrin-α, a p53-specific inhibitor. Ectopic expression of Bcl-2 also inhibited apoptosis in NVP-BE235 plus curcumin-treated cells. In contrast, NVP-BEZ235 combined with curcumin did not have a synergistic effect on normal human skin fibroblasts and normal human mesangial cells. Taken together, combined treatment with NVP-BEZ235 and curcumin induces apoptosis through p53-dependent Bcl-2 mRNA down-regulation at the transcriptional level and Mcl-1 protein down-regulation at the post-transcriptional level.
Conflict of interest statement
Figures




References
-
- Sun X, Huang J, Homma T, Kita D, Klocker H, et al. (2009) Genetic alterations in the PI3K pathway in prostate cancer. Anticancer research 29: 1739–1743. - PubMed
-
- Tang JM, He QY, Guo RX, Chang XJ (2006) Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung cancer 51: 181–191. - PubMed
-
- Altomare DA, Testa JR (2005) Perturbations of the AKT signaling pathway in human cancer. Oncogene 24: 7455–7464. - PubMed
-
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, et al. (2007) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316: 1039–1043. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

