The developing story of Sprouty and cancer
- PMID: 24744103
- PMCID: PMC4113681
- DOI: 10.1007/s10555-014-9497-1
The developing story of Sprouty and cancer
Abstract
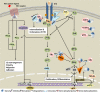
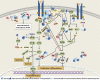
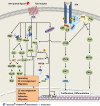
Sprouty proteins are evolutionarily conserved modulators of MAPK/ERK pathway. Through interacting with an increasing number of effectors, mediators, and regulators with ultimate influence on multiple targets within or beyond ERK, Sprouty orchestrates a complex, multilayered regulatory system and mediates a crosstalk among different signaling pathways for a coordinated cellular response. As such, Sprouty has been implicated in various developmental and physiological processes. Evidence shows that ERK is aberrantly activated in malignant conditions. Accordingly, Sprouty deregulation has been reported in different cancer types and shown to impact cancer development, progression, and metastasis. In this article, we have tried to provide an overview of the current knowledge about the Sprouty physiology and its regulatory functions in health, as well as an updated review of the Sprouty status in cancer. Putative implications of Sprouty in cancer biology, their clinical relevance, and their proposed applications are also revisited. As a developing story, however, role of Sprouty in cancer remains to be further elucidated.
Figures
References
-
- Hacohen N, Kramer S, Sutherland D, Hiromi Y, Krasnow MA. Sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92(2):253–263. - PubMed
-
- Kramer S, Okabe M, Hacohen N, Krasnow MA, Hiromi Y. Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] Development. 1999;126(11):2515–2525. - PubMed
-
- de Maximy AA, Nakatake Y, Moncada S, Itoh N, Thiery JP, Bellusci S. Cloning and expression pattern of a mouse homologue of drosophila sprouty in the mouse embryo. [Research Support, Non-U.S. Gov't] Mechanisms of Development. 1999;81(1–2):213–216. - PubMed
-
- Leeksma OC, Van Achterberg TA, Tsumura Y, Toshima J, Eldering E, Kroes WG, et al. Human sprouty 4, a new ras antagonist on 5q31, interacts with the dual specificity kinase TESK1. European Journal of Biochemistry. 2002;269(10):2546–2556. - PubMed
-
- Gross I, Bassit B, Benezra M, Licht JD. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. Journal of Biological Chemistry. 2001;276(49):46460–46468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

