DNA-protein π-interactions in nature: abundance, structure, composition and strength of contacts between aromatic amino acids and DNA nucleobases or deoxyribose sugar
- PMID: 24744240
- PMCID: PMC4041443
- DOI: 10.1093/nar/gku269
DNA-protein π-interactions in nature: abundance, structure, composition and strength of contacts between aromatic amino acids and DNA nucleobases or deoxyribose sugar
Abstract
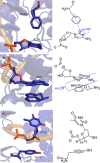
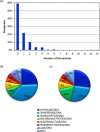
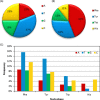
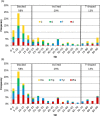
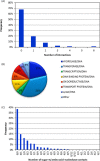
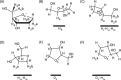
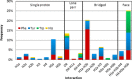
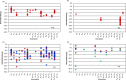
Four hundred twenty-eight high-resolution DNA-protein complexes were chosen for a bioinformatics study. Although 164 crystal structures (38% of those searched) contained no interactions, 574 discrete π-contacts between the aromatic amino acids and the DNA nucleobases or deoxyribose were identified using strict criteria, including visual inspection. The abundance and structure of the interactions were determined by unequivocally classifying the contacts as either π-π stacking, π-π T-shaped or sugar-π contacts. Three hundred forty-four nucleobase-amino acid π-π contacts (60% of all interactions identified) were identified in 175 of the crystal structures searched. Unprecedented in the literature, 230 DNA-protein sugar-π contacts (40% of all interactions identified) were identified in 137 crystal structures, which involve C-H···π and/or lone-pair···π interactions, contain any amino acid and can be classified according to sugar atoms involved. Both π-π and sugar-π interactions display a range of relative monomer orientations and therefore interaction energies (up to -50 (-70) kJ mol(-1) for neutral (charged) interactions as determined using quantum chemical calculations). In general, DNA-protein π-interactions are more prevalent than perhaps currently accepted and the role of such interactions in many biological processes may yet to be uncovered.
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

