Iron-containing micronutrient supplementation of Chinese women with no or mild anemia during pregnancy improved iron status but did not affect perinatal anemia
- PMID: 24744317
- PMCID: PMC9035907
- DOI: 10.3945/jn.113.189894
Iron-containing micronutrient supplementation of Chinese women with no or mild anemia during pregnancy improved iron status but did not affect perinatal anemia
Abstract
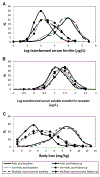
Universal prenatal daily iron-folic acid (IFA) and multiple micronutrient (MM) supplements are recommended to reduce the risk of low birth weight, maternal anemia, and iron deficiency (ID) during pregnancy, but the evidence of their effect on iron status among women with mild or no anemia is limited. The aim of this study was to describe the iron status [serum ferritin (SF), serum soluble transferrin receptor (sTfR), and body iron (BI)] before and after micronutrient supplementation during pregnancy. We examined 834 pregnant women with hemoglobin > 100 g/L at enrollment before 20 wk of gestation and with iron measurement data from a subset of a randomized, double-blind trial in China. Women were randomly assigned to take daily 400 μg of folic acid (FA) (control), FA plus 30 mg of iron, or FA, iron, plus 13 additional MMs provided before 20 wk of gestation to delivery. Venous blood was collected in this subset during study enrollment (before 20 wk of gestation) and 28-32 wk of gestation. We found that, at 28-32 wk of gestation, compared with the FA group, both the IFA and MM groups had significantly lower prevalence of ID regardless of which indicator (SF, sTfR, or BI) was used for defining ID. The prevalence of ID at 28-32 wk of gestation for IFA, MM, and FA were 35.3%, 42.7%, and 59.6% by using low SF, 53.6%, 59.9%, and 69.9% by using high sTfR, and 34.5%, 41.2%, and 59.6% by using low BI, respectively. However, there was no difference in anemia prevalence (hemoglobin < 110 g/L) between FA and IFA or MM groups. We concluded that, compared with FA alone, prenatal IFA and MM supplements provided to women with no or mild anemia improved iron status later during pregnancy but did not affect perinatal anemia. This trial was registered at clinicaltrials.gov as NCT00137744.
© 2014 American Society for Nutrition.
Conflict of interest statement
Author disclosures: Z. Mei, M. K. Serdula, J.-m. Liu, R. C. Flores-Ayala, L. Wang, R. Ye, and L. M. Grummer-Strawn, no conflicts of interest,
Figures

References
-
- World Health Organization. Guideline: daily iron and folic acid supplementation in pregnant women. Geneva, Switzerland: WHO, 2012. - PubMed
-
- Allen LH, Peerson JM; Maternal Micronutrient Supplementation Study Group. Impact of multiple micronutrient versus iron-folic acid supplements on maternal anemia and micronutrient status in pregnancy. Food Nutr Bull 2009;30(Suppl 4):S527–32. - PubMed
-
- Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep 1998;47:1–29. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

