Aldosterone regulates microRNAs in the cortical collecting duct to alter sodium transport
- PMID: 24744440
- PMCID: PMC4214524
- DOI: 10.1681/ASN.2013090931
Aldosterone regulates microRNAs in the cortical collecting duct to alter sodium transport
Erratum in
-
Erratum.J Am Soc Nephrol. 2015 Jun;26(6):1476. doi: 10.1681/ASN.2015040401. J Am Soc Nephrol. 2015. PMID: 26025913 Free PMC article. No abstract available.
-
Erratum.J Am Soc Nephrol. 2015 Oct;26(10):2600. doi: 10.1681/ASN.2015070789. J Am Soc Nephrol. 2015. PMID: 26424868 Free PMC article. No abstract available.
Abstract
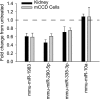
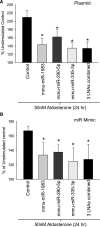
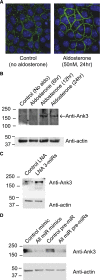
A role for microRNAs (miRs) in the physiologic regulation of sodium transport in the kidney has not been established. In this study, we investigated the potential of aldosterone to alter miR expression in mouse cortical collecting duct (mCCD) epithelial cells. Microarray studies demonstrated the regulation of miR expression by aldosterone in both cultured mCCD and isolated primary distal nephron principal cells. Aldosterone regulation of the most significantly downregulated miRs, mmu-miR-335-3p, mmu-miR-290-5p, and mmu-miR-1983 was confirmed by quantitative RT-PCR. Reducing the expression of these miRs separately or in combination increased epithelial sodium channel (ENaC)-mediated sodium transport in mCCD cells, without mineralocorticoid supplementation. Artificially increasing the expression of these miRs by transfection with plasmid precursors or miR mimic constructs blunted aldosterone stimulation of ENaC transport. Using a newly developed computational approach, termed ComiR, we predicted potential gene targets for the aldosterone-regulated miRs and confirmed ankyrin 3 (Ank3) as a novel aldosterone and miR-regulated protein. A dual-luciferase assay demonstrated direct binding of the miRs with the Ank3-3' untranslated region. Overexpression of Ank3 increased and depletion of Ank3 decreased ENaC-mediated sodium transport in mCCD cells. These findings implicate miRs as intermediaries in aldosterone signaling in principal cells of the distal kidney nephron.
Copyright © 2014 by the American Society of Nephrology.
Figures





References
-
- Garty H, Palmer LG: Epithelial sodium channels: Function, structure, and regulation. Physiol Rev 77: 359–396, 1997 - PubMed
-
- Kellenberger S, Schild L: Epithelial sodium channel/degenerin family of ion channels: A variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 - PubMed
-
- Bhalla V, Hallows KR: Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol 19: 1845–1854, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 DK087922/DK/NIDDK NIH HHS/United States
- R01 DK047874/DK/NIDDK NIH HHS/United States
- P30-CA47904/CA/NCI NIH HHS/United States
- P30-DK079307/DK/NIDDK NIH HHS/United States
- R01-DK047874/DK/NIDDK NIH HHS/United States
- R01 DK091565/DK/NIDDK NIH HHS/United States
- R00-DK078917/DK/NIDDK NIH HHS/United States
- R01 LM009657/LM/NLM NIH HHS/United States
- R00-DK087922/DK/NIDDK NIH HHS/United States
- P30 DK079307/DK/NIDDK NIH HHS/United States
- R01-LM009657/LM/NLM NIH HHS/United States
- R00 DK078917/DK/NIDDK NIH HHS/United States
- P30 CA047904/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

