Immunophenotype of normal and myelomatous plasma-cell subsets
- PMID: 24744760
- PMCID: PMC3978250
- DOI: 10.3389/fimmu.2014.00137
Immunophenotype of normal and myelomatous plasma-cell subsets
Abstract
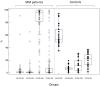
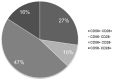
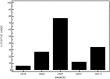
Plasma cells (PCs) are essentially characterized by the co-expression of CD138 and CD38, which allows their identification in flow cytometry in bone marrow (BM), peripheral blood, or cell suspensions from tissues. These terminally differentiated B-cells may lose the expression of surface CD19 and that of CD20 while retaining CD27. When malignant, they can gain a number of other markers such as CD28, CD33, CD56, or CD117 and lose CD27. Moreover, since each PC is only able to produce a single type of immunoglobulins (Igs), they display isotypic restriction and clonal malignant PCs can be further characterized by their homogeneous expression of either kappa or lambda light chains. In multiple myeloma (MM), such PC clones produce the Ig identified in plasma as an abnormal peak. In the BM where they essentially accumulate, these PCs may however display various immunophenotypes. The latter were explored in a two-way approach. Firstly, the various subsets delineated by the selective or common expression of CD19 together with combined CD56/CD28 were explored in normal and MM BM. Then, other aberrant markers' expression was investigated, i.e., CD20, CD27, CD33, CD56, CD117. These data were compared to literature information. They underline the vast heterogeneity of MM PCs possibly accounting for the various answers to therapy of MM patients.
Keywords: bone marrow; flow cytometry; immunophenotype; multiple myeloma; plasma cells.
Figures


References
-
- Bataille R, Jégo G, Robillard N, Barillé-Nion S, Harousseau JL, Moreau P, et al. The phenotype of normal, reactive and malignant plasma cells. Identification of “many and multiple myelomas” and of new targets for myeloma therapy. Haematologica (2006) 91:1234–40 - PubMed
-
- Cannizzo E, Bellio E, Sohani AR, Hasserjian RP, Ferry JA, Dorn ME, et al. Multiparameter immunophenotyping by flow cytometry in multiple myeloma: the diagnostic utility of defining ranges of normal antigenic expression in comparison to histology. Cytometry B Clin Cytom (2010) 78:231–8 10.1002/cyto.b.20517 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

