Effects of gravitational loading levels on protein expression related to metabolic and/or morphologic properties of mouse neck muscles
- PMID: 24744868
- PMCID: PMC3967672
- DOI: 10.1002/phy2.183
Effects of gravitational loading levels on protein expression related to metabolic and/or morphologic properties of mouse neck muscles
Abstract
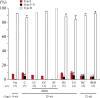
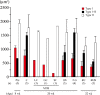
The effects of 3 months of spaceflight (SF), hindlimb suspension, or exposure to 2G on the characteristics of neck muscle in mice were studied. Three 8-week-old male C57BL/10J wild-type mice were exposed to microgravity on the International Space Station in mouse drawer system (MDS) project, although only one mouse returned to the Earth alive. Housing of mice in a small MDS cage (11.6 × 9.8-cm and 8.4-cm height) and/or in a regular vivarium cage was also performed as the ground controls. Furthermore, ground-based hindlimb suspension and 2G exposure by using animal centrifuge (n = 5 each group) were performed. SF-related shift of fiber phenotype from type I to II and atrophy of type I fibers were noted. Shift of fiber phenotype was related to downregulation of mitochondrial proteins and upregulation of glycolytic proteins, suggesting a shift from oxidative to glycolytic metabolism. The responses of proteins related to calcium handling, myofibrillar structure, and heat stress were also closely related to the shift of muscular properties toward fast-twitch type. Surprisingly, responses of proteins to 2G exposure and hindlimb suspension were similar to SF, although the shift of fiber types and atrophy were not statistically significant. These phenomena may be related to the behavior of mice that the relaxed posture without lifting their head up was maintained after about 2 weeks. It was suggested that inhibition of normal muscular activities associated with gravitational unloading causes significant changes in the protein expression related to metabolic and/or morphological properties in mouse neck muscle.
Keywords: gravitational unloading; mouse neck muscle; protein expression.
Figures



References
-
- Alford E. K., Roy R. R., Hodgson J. A., Edgerton V. R. 1987. Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hindlimb suspension. Exp. Neurol.; 96:635-649 - PubMed
-
- Atomi Y., Yamada S., Strohman R., Nonomura Y. 1991. Alpha B‐crystallin in skeletal muscle: purification and localization. J. Biochem.; 110:812-822 - PubMed
-
- Berman Y., North K. N. 2010. A gene for speed: the emerging role of alpha‐actinin‐3 in muscle metabolism. Physiology (Bethesda); 25:250-259 - PubMed
-
- Caiozzo V. J., Baker M. J., Herrick R. E., Tao M., Baldwin K. M. 1994. Effects of spaceflight on skeletal muscle: mechanical properties and myosin isoform content of slow muscle. J. Appl. Physiol.; 76:1764-1773 - PubMed
-
- Cancedda R., Pignataro S., Alberici G., Tenconi C. 2002. Mice Drawer System: phase c/d development and perspective. J. Gravit. Physiol.; 9:P337-P338 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

