Parathyroid hormone secretion by multiple distinct cell populations, a time dynamic mathematical model
- PMID: 24744900
- PMCID: PMC3966243
- DOI: 10.1002/phy2.231
Parathyroid hormone secretion by multiple distinct cell populations, a time dynamic mathematical model
Abstract
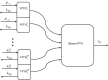
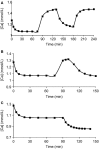
The acute response of parathyroid hormone to perturbations in serum ionized calcium ([Ca(2+)]) is physiologically complex, and poorly understood. The literature provides numerous observations of quantitative and qualitative descriptions of parathyroid hormone (PTH) dynamics. We present a physiologically based mathematical model of PTH secretion constructed from mechanisms suggested in the literature, and validated against complex [Ca(2+)] clamping protocols from human data. The model is based on two assumptions. The first is that secretion is a fraction of cellular reserves, with the fraction being determined by the kinetics of [Ca(2+)] with its receptor. The second is that there are multiple distinct populations of parathyroid cells, with different secretory parameters. The steady state and transient PTH secretion responses of the model are in agreement with human experimental PTH responses to different hypocalcemia and hypercalcemia stimuli. This mathematical model suggests that a population of secreting cells is responsible for the PTH secretory dynamics observed experimentally.
Keywords: Calcium; hysteresis; parathyroid hormone; simulation.
Figures



Similar articles
-
PTHrP enhances the secretory response of PTH to a hypocalcemic stimulus in rat parathyroid glands.Kidney Int. 2000 Jul;58(1):71-81. doi: 10.1046/j.1523-1755.2000.00142.x. Kidney Int. 2000. PMID: 10886551
-
Hysteresis of the parathyroid hormone response to hypocalcemia in hemodialysis patients with low turnover aluminum bone disease.J Am Soc Nephrol. 1991 Dec;2(6):1136-43. doi: 10.1681/ASN.V261136. J Am Soc Nephrol. 1991. PMID: 1777594
-
Control of pulsatile and tonic parathyroid hormone secretion by ionized calcium.J Clin Endocrinol Metab. 1996 Dec;81(12):4236-43. doi: 10.1210/jcem.81.12.8954021. J Clin Endocrinol Metab. 1996. PMID: 8954021
-
In vivo studies of parathyroid gland function in secondary hyperparathyroidism.Adv Nephrol Necker Hosp. 1996;25:289-302. Adv Nephrol Necker Hosp. 1996. PMID: 8717632 Review.
-
Humoral hypercalcemia associated with gastric carcinoma secreting parathyroid hormone: a case report and review of the literature.Endocr J. 2013;60(5):557-62. doi: 10.1507/endocrj.ej12-0406. Epub 2013 Jan 9. Endocr J. 2013. PMID: 23303131 Review.
References
-
- Agus Z. S., Gardner L. B., Beck L. H., Goldberg M. 1973. Effects of parathyroid hormone on renal tubular reabsorption of calcium, sodium, and phosphate. Am. J. Physiol.; 224:1143-1148 - PubMed
-
- Almaden Y., Canalejo A., Hernandez A., Ballesteros E., Garcia‐Navarro S., Torres A. 1996. Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J. Bone Miner. Res.; 11:970-976 - PubMed
-
- Bas S., Aguilera‐Tejero E., Estepa J. C., Garfia B., Lopez I., Rodriguez M. 2002. The influence of acute and chronic hypercalcemia on the parathyroid hormone response to hypocalcemia in rabbits. Eur. J. Endocrinol.; 146:411-418 - PubMed
-
- Brown E. M. 1983. Four‐parameter model of the sigmoidal relationship between parathyroid hormone release and extracellular calcium concentration in normal and abnormal parathyroid tissue. J. Clin. Endocrinol. Metab.; 56:572-581 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

