The integrative role of the sigh in psychology, physiology, pathology, and neurobiology
- PMID: 24746045
- PMCID: PMC4427060
- DOI: 10.1016/B978-0-444-63274-6.00006-0
The integrative role of the sigh in psychology, physiology, pathology, and neurobiology
Abstract
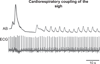
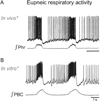
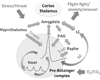
"Sighs, tears, grief, distress" expresses Johann Sebastian Bach in a musical example for the relationship between sighs and deep emotions. This review explores the neurobiological basis of the sigh and its relationship with psychology, physiology, and pathology. Sighs monitor changes in brain states, induce arousal, and reset breathing variability. These behavioral roles homeostatically regulate breathing stability under physiological and pathological conditions. Sighs evoked in hypoxia evoke arousal and thereby become critical for survival. Hypoarousal and failure to sigh have been associated with sudden infant death syndrome. Increased breathing irregularity may provoke excessive sighing and hyperarousal, a behavioral sequence that may play a role in panic disorders. Essential for generating sighs and breathing is the pre-Bötzinger complex. Modulatory and synaptic interactions within this local network and between networks located in the brainstem, cerebellum, cortex, hypothalamus, amygdala, and the periaqueductal gray may govern the relationships between physiology, psychology, and pathology. Unraveling these circuits will lead to a better understanding of how we balance emotions and how emotions become pathological.
Keywords: PAG; SIDS; anxiety; arousal; breathing; cardiorespiratory; panic; pre-Bötzinger complex; rhythm generation.
© 2014 Elsevier B.V. All rights reserved.
Figures






References
-
- Abelson JL, Nesse RM, Weg JG, Curtis GC. Respiratory psychophysiology and anxiety: cognitive intervention in the doxapram model of panic. Psychosom. Med. 1996;58:302–313. - PubMed
-
- Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biol. Psychiatry. 2001;49:588–595. - PubMed
-
- Abelson JL, Khan S, Lyubkin M, Giardino N. Respiratory irregularity and stress hormones in panic disorder: exploring potential linkages. Depress. Anxiety. 2008;25:885–887. - PubMed
-
- Abubakr A, Ifeayni I, Wambacq I. The efficacy of routine hyperventilation for seizure activation during prolonged video-electroencephalography monitoring. J. Clin. Neurosci. 2010;17:1503–1505. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

