Slow and fast γ rhythms coordinate different spatial coding modes in hippocampal place cells
- PMID: 24746420
- PMCID: PMC4109650
- DOI: 10.1016/j.neuron.2014.03.013
Slow and fast γ rhythms coordinate different spatial coding modes in hippocampal place cells
Abstract
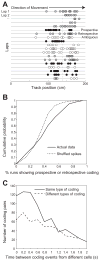
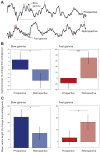
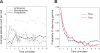
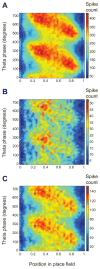
Previous work has hinted that prospective and retrospective coding modes exist in hippocampus. Prospective coding is believed to reflect memory retrieval processes, whereas retrospective coding is thought to be important for memory encoding. Here, we show in rats that separate prospective and retrospective modes exist in hippocampal subfield CA1 and that slow and fast gamma rhythms differentially coordinate place cells during the two modes. Slow gamma power and phase locking of spikes increased during prospective coding; fast gamma power and phase locking increased during retrospective coding. Additionally, slow gamma spikes occurred earlier in place fields than fast gamma spikes, and cell ensembles retrieved upcoming positions during slow gamma and encoded past positions during fast gamma. These results imply that alternating slow and fast gamma states allow the hippocampus to switch between prospective and retrospective modes, possibly to prevent interference between memory retrieval and encoding.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

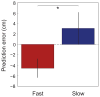
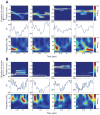
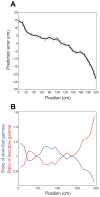
Comment in
-
On track with two gammas.Neuron. 2014 May 7;82(3):506-8. doi: 10.1016/j.neuron.2014.04.027. Neuron. 2014. PMID: 24811375
References
-
- Barbieri R, Wilson MA, Frank LM, Brown EN. An analysis of hippocampal spatio-temporal representations using a Bayesian Algorithm for Neural Spike Train Decoding. IEEE Trans Neural Syst Rehabil Eng. 2005;13:131–136. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

