Inducible nitric oxide synthase is key to peroxynitrite-mediated, LPS-induced protein radical formation in murine microglial BV2 cells
- PMID: 24746617
- PMCID: PMC4111989
- DOI: 10.1016/j.freeradbiomed.2014.04.014
Inducible nitric oxide synthase is key to peroxynitrite-mediated, LPS-induced protein radical formation in murine microglial BV2 cells
Abstract
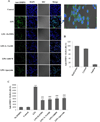
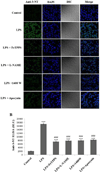
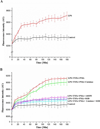
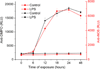
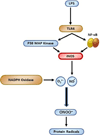
Microglia are the resident immune cells in the brain. Microglial activation is characteristic of several inflammatory and neurodegenerative diseases including Alzheimer's disease, multiple sclerosis, and Parkinson's disease. Though lipopolysaccharide (LPS)-induced microglial activation in models of Parkinson's disease is well documented, the free radical-mediated protein radical formation and its underlying mechanism during LPS-induced microglial activation are not known. Here we have used immuno-spin trapping and RNA interference to investigate the role of inducible nitric oxide synthase (iNOS) in peroxynitrite-mediated protein radical formation in murine microglial BV2 cells treated with LPS. Treatment of BV2 cells with LPS resulted in morphological changes, induction of iNOS, and increased protein radical formation. Pretreatments with FeTPPS (a peroxynitrite decomposition catalyst), L-NAME (total NOS inhibitor), 1400W (iNOS inhibitor), and apocynin significantly attenuated LPS-induced protein radical formation and tyrosine nitration. Results obtained with coumarin-7-boronic acid, a highly specific probe for peroxynitrite detection, correlated with LPS-induced tyrosine nitration, which demonstrated involvement of peroxynitrite in protein radical formation. A similar degree of protection conferred by 1400W and L-NAME led us to conclude that only iNOS, and no other forms of NOS, is involved in LPS-induced peroxynitrite formation. Subsequently, siRNA for iNOS, the iNOS-specific inhibitor 1400W, the NF-κB inhibitor PDTC, and the p38 MAPK inhibitor SB202190 was used to inhibit iNOS directly or indirectly. Inhibition of iNOS precisely correlated with decreased protein radical formation in LPS-treated BV2 cells. The time course of protein radical formation also matched the time course of iNOS expression. Taken together, these results prove the role of iNOS in peroxynitrite-mediated protein radical formation in LPS-treated microglial BV2 cells.
Keywords: Free radicals; Inducible nitric oxide synthase; Lipopolysaccharide; Microglia; Nitrone adducts; Parkinson disease; Peroxynitrite; Protein radical.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


References
-
- Bezard E, Przedborski S. A tale on animal models of Parkinson's disease. Mov. Disord. 2011;26:993–1002. - PubMed
-
- Li M, Dai FR, Du XP, Yang QD, Chen Y. Neuroprotection by silencing iNOS expression in a 6-OHDA model of Parkinson's disease. J. Mol. Neurosci. 2012;48:225–233. - PubMed
-
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

