(-)-Epicatechin mitigates high-fructose-associated insulin resistance by modulating redox signaling and endoplasmic reticulum stress
- PMID: 24746618
- PMCID: PMC4077617
- DOI: 10.1016/j.freeradbiomed.2014.04.011
(-)-Epicatechin mitigates high-fructose-associated insulin resistance by modulating redox signaling and endoplasmic reticulum stress
Abstract
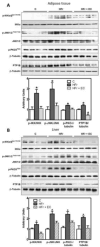
We investigated the capacity of dietary (-)-epicatechin (EC) to mitigate insulin resistance through the modulation of redox-regulated mechanisms in a rat model of metabolic syndrome. Adolescent rats were fed a regular chow diet without or with high fructose (HFr; 10% w/v) in drinking water for 8 weeks, and a group of HFr-fed rats was supplemented with EC in the diet. HFr-fed rats developed insulin resistance, which was mitigated by EC supplementation. Accordingly, the activation of components of the insulin signaling cascade (insulin receptor, IRS1, Akt, and ERK1/2) was impaired, whereas negative regulators (PKC, IKK, JNK, and PTP1B) were upregulated in the liver and adipose tissue of HFr rats. These alterations were partially or totally prevented by EC supplementation. In addition, EC inhibited events that contribute to insulin resistance: HFr-associated increased expression and activity of NADPH oxidase, activation of redox-sensitive signals, expression of NF-κB-regulated proinflammatory cytokines and chemokines, and some sub-arms of endoplasmic reticulum stress signaling. Collectively, these findings indicate that EC supplementation can mitigate HFr-induced insulin resistance and are relevant for defining interventions that can prevent/mitigate MetS-associated insulin resistance.
Keywords: Endoplasmic reticulum stress; Epicatechin and flavonoids; Free radicals; Insulin resistance; Metabolic syndrome; NADPH oxidase; Redox signaling.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
(-)-Epicatechin improves insulin sensitivity in high fat diet-fed mice.Arch Biochem Biophys. 2016 Jun 1;599:13-21. doi: 10.1016/j.abb.2016.03.006. Epub 2016 Mar 8. Arch Biochem Biophys. 2016. PMID: 26968772 Free PMC article.
-
(-)-Epicatechin reduces blood pressure increase in high-fructose-fed rats: effects on the determinants of nitric oxide bioavailability.J Nutr Biochem. 2015 Jul;26(7):745-51. doi: 10.1016/j.jnutbio.2015.02.004. Epub 2015 Mar 27. J Nutr Biochem. 2015. PMID: 25943039
-
Dietary (-)-epicatechin mitigates oxidative stress, NO metabolism alterations, and inflammation in renal cortex from fructose-fed rats.Free Radic Biol Med. 2016 Jan;90:35-46. doi: 10.1016/j.freeradbiomed.2015.11.009. Epub 2015 Nov 10. Free Radic Biol Med. 2016. PMID: 26569027
-
(-)-Epicatechin in the control of glucose homeostasis: Involvement of redox-regulated mechanisms.Free Radic Biol Med. 2019 Jan;130:478-488. doi: 10.1016/j.freeradbiomed.2018.11.010. Epub 2018 Nov 14. Free Radic Biol Med. 2019. PMID: 30447350 Review.
-
Dietary omega-3 fatty acid deficiency and high fructose intake in the development of metabolic syndrome, brain metabolic abnormalities, and non-alcoholic fatty liver disease.Nutrients. 2013 Jul 26;5(8):2901-23. doi: 10.3390/nu5082901. Nutrients. 2013. PMID: 23896654 Free PMC article. Review.
Cited by
-
LPS-induced renal inflammation is prevented by (-)-epicatechin in rats.Redox Biol. 2017 Apr;11:342-349. doi: 10.1016/j.redox.2016.12.023. Epub 2016 Dec 22. Redox Biol. 2017. PMID: 28039839 Free PMC article.
-
Epicatechin Influence on Biochemical Modification of Human Erythrocyte Metabolism and Membrane Integrity.Int J Mol Sci. 2024 Dec 16;25(24):13481. doi: 10.3390/ijms252413481. Int J Mol Sci. 2024. PMID: 39769244 Free PMC article.
-
Red rice bran aqueous extract ameliorate diabetic status by inhibiting intestinal glucose transport in high fat diet/STZ-induced diabetic rats.J Tradit Complement Med. 2023 Dec 25;14(4):391-402. doi: 10.1016/j.jtcme.2023.12.003. eCollection 2024 Jul. J Tradit Complement Med. 2023. PMID: 39035687 Free PMC article.
-
Age-Dependent Skeletal Muscle Mitochondrial Response to Short-Term Increased Dietary Fructose.Antioxidants (Basel). 2023 Jan 28;12(2):299. doi: 10.3390/antiox12020299. Antioxidants (Basel). 2023. PMID: 36829857 Free PMC article.
-
NADPH Oxidases Connecting Fatty Liver Disease, Insulin Resistance and Type 2 Diabetes: Current Knowledge and Therapeutic Outlook.Antioxidants (Basel). 2022 Jun 9;11(6):1131. doi: 10.3390/antiox11061131. Antioxidants (Basel). 2022. PMID: 35740032 Free PMC article. Review.
References
-
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. - PubMed
-
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. - PubMed
-
- Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–375. - PubMed
-
- Fraga CG, Oteiza PI. Dietary flavonoids: Role of (-)-epicatechin and related procyanidins in cell signaling. Free Radic Biol Med. 2011;51:813–823. - PubMed
-
- Hostmark AT. The Oslo Health Study: a Dietary Index estimating high intake of soft drinks and low intake of fruits and vegetables was positively associated with components of the metabolic syndrome. Appl Physiol Nutr Metab. 2010;35:816–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

