proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus
- PMID: 24746813
- PMCID: PMC4118923
- DOI: 10.1016/j.celrep.2014.03.040
proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus
Abstract
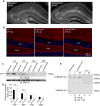
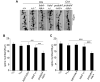
Experience-dependent plasticity shapes postnatal development of neural circuits, but the mechanisms that refine dendritic arbors, remodel spines, and impair synaptic activity are poorly understood. Mature brain-derived neurotrophic factor (BDNF) modulates neuronal morphology and synaptic plasticity, including long-term potentiation (LTP) via TrkB activation. BDNF is initially translated as proBDNF, which binds p75(NTR). In vitro, recombinant proBDNF modulates neuronal structure and alters hippocampal long-term plasticity, but the actions of endogenously expressed proBDNF are unclear. Therefore, we generated a cleavage-resistant probdnf knockin mouse. Our results demonstrate that proBDNF negatively regulates hippocampal dendritic complexity and spine density through p75(NTR). Hippocampal slices from probdnf mice exhibit depressed synaptic transmission, impaired LTP, and enhanced long-term depression (LTD) in area CA1. These results suggest that proBDNF acts in vivo as a biologically active factor that regulates hippocampal structure, synaptic transmission, and plasticity, effects that are distinct from those of mature BDNF.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



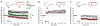
References
-
- Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Curr Opin Neurobiol. 2008;18:524–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

