The contribution of co-transcriptional RNA:DNA hybrid structures to DNA damage and genome instability
- PMID: 24746923
- PMCID: PMC4051866
- DOI: 10.1016/j.dnarep.2014.03.023
The contribution of co-transcriptional RNA:DNA hybrid structures to DNA damage and genome instability
Abstract
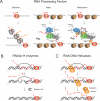
Accurate DNA replication and DNA repair are crucial for the maintenance of genome stability, and it is generally accepted that failure of these processes is a major source of DNA damage in cells. Intriguingly, recent evidence suggests that DNA damage is more likely to occur at genomic loci with high transcriptional activity. Furthermore, loss of certain RNA processing factors in eukaryotic cells is associated with increased formation of co-transcriptional RNA:DNA hybrid structures known as R-loops, resulting in double-strand breaks (DSBs) and DNA damage. However, the molecular mechanisms by which R-loop structures ultimately lead to DNA breaks and genome instability is not well understood. In this review, we summarize the current knowledge about the formation, recognition and processing of RNA:DNA hybrids, and discuss possible mechanisms by which these structures contribute to DNA damage and genome instability in the cell.
Keywords: APOBEC family; ASF splicing factor; Activation-induced deaminase (AID); Double-strand breaks; G-quadruplex; Genome instability; R-loops; RNA processing factors; RNA:DNA helicases; RNase H; Senataxin; THO/TREX complex; THSC/TREX-2; Topoisomerase; Transcription–replication conflicts; mRNP biogenesis.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures



References
-
- Aguilera A, García-Muse T. R loops: from transcription byproducts to threats to genome stability. Mol. Cell. 2012;46:115–124. - PubMed
-
- Aguilera A, García-Muse T. Causes of genome instability. Annu. Rev. Genet. 2013;47:1–32. - PubMed
-
- Aguilera A, Gómez-González B. Genome instability: a mechanistic view of its causes and consequences. Nat. Rev. Genet. 2008;9:204–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

