An overview of clinical and commercial impact of drug delivery systems
- PMID: 24747160
- PMCID: PMC4142089
- DOI: 10.1016/j.jconrel.2014.03.053
An overview of clinical and commercial impact of drug delivery systems
Abstract
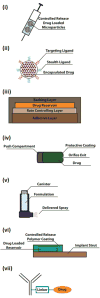
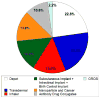
Drug delivery systems are widely researched and developed to improve the delivery of pharmaceutical compounds and molecules. The last few decades have seen a marked growth of the field fueled by increased number of researchers, research funding, venture capital and the number of start-ups. Collectively, the growth has led to novel systems that make use of micro/nano-particles, transdermal patches, inhalers, drug reservoir implants and antibody-drug conjugates. While the increased research activity is clearly an indication of proliferation of the field, clinical and commercial translation of early-stage research ideas is critically important for future growth and interest in the field. Here, we will highlight some of the examples of novel drug delivery systems that have undergone such translation. Specifically, we will discuss the developments, advantages, limitations and lessons learned from: (i) microparticle-based depot formulations, (ii) nanoparticle-based cancer drugs, (iii) transdermal systems, (iv) oral drug delivery systems, (v) pulmonary drug delivery, (vi) implants and (vii) antibody-drug conjugates. These systems have impacted treatment of many prevalent diseases including diabetes, cancer and cardiovascular diseases, among others. At the same time, these systems are integral and enabling components of products that collectively generate annual revenues exceeding US $100 billion. These examples provide strong evidence of the clinical and commercial impact of drug delivery systems.
Keywords: Clinical translation; Drug delivery; Historical; Lab to clinic; Perspective; Pharmaceutics.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

References
-
- Kermode M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health promotion international. 2004;19(1):95–103. - PubMed
-
- Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2(4):289–95. - PubMed
-
- Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6(1):67–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

