Trypanosoma cruzi bromodomain factor 3 binds acetylated α-tubulin and concentrates in the flagellum during metacyclogenesis
- PMID: 24747213
- PMCID: PMC4054268
- DOI: 10.1128/EC.00341-13
Trypanosoma cruzi bromodomain factor 3 binds acetylated α-tubulin and concentrates in the flagellum during metacyclogenesis
Abstract
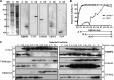
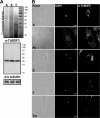
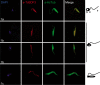
Bromodomains are highly conserved acetyl-lysine binding domains found mainly in proteins associated with chromatin and nuclear acetyltransferases. The Trypanosoma cruzi genome encodes at least four bromodomain factors (TcBDFs). We describe here bromodomain factor 3 (TcBDF3), a bromodomain-containing protein localized in the cytoplasm. TcBDF3 cytolocalization was determined, using purified antibodies, by Western blot and immunofluorescence analyses in all life cycle stages of T. cruzi. In epimastigotes and amastigotes, it was detected in the cytoplasm, the flagellum, and the flagellar pocket, and in trypomastigotes only in the flagellum. Subcellular localization of TcBDF3 was also determined by digitonin extraction, ultrastructural immunocytochemistry, and expression of TcBDF3 fused to cyan fluorescent protein (CFP). Tubulin can acquire different posttranslational modifications, which modulate microtubule functions. Acetylated α-tubulin has been found in the axonemes of flagella and cilia, as well as in the subpellicular microtubules of trypanosomatids. TcBDF3 and acetylated α-tubulin partially colocalized in isolated cytoskeletons and flagella from T. cruzi epimastigotes and trypomastigotes. Interaction between the two proteins was confirmed by coimmunoprecipitation and far-Western blot assays with synthetic acetylated α-tubulin peptides and recombinant TcBDF3.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

