Cortical neurogenesis from pluripotent stem cells: complexity emerging from simplicity
- PMID: 24747604
- PMCID: PMC4386058
- DOI: 10.1016/j.conb.2014.03.012
Cortical neurogenesis from pluripotent stem cells: complexity emerging from simplicity
Abstract
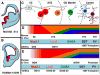
The cerebral cortex contains dozens of neuronal subtypes grouped in specific layers and areas. Recent studies have revealed how embryonic and induced pluripotent stem cells (PSC) can differentiate into a wide diversity of cortical neurons in vitro, while recapitulating many of the temporal and spatial features that characterize corticogenesis. PSC-derived neurons can integrate into the brain following in vivo transplantation and display patterns of morphology and connectivity specific of cortical neurons. PSC-corticogenesis thus emerges as a robust model that provides new ways to link cortical development, evolution, and disease.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
-
- Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. - PubMed
-
- Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

