Anti-proliferative and apoptosis-inducing effects of camptothecin-20(s)-O-(2-pyrazolyl-1)acetic ester in human breast tumor MCF-7 cells
- PMID: 24747650
- PMCID: PMC6271214
- DOI: 10.3390/molecules19044941
Anti-proliferative and apoptosis-inducing effects of camptothecin-20(s)-O-(2-pyrazolyl-1)acetic ester in human breast tumor MCF-7 cells
Abstract
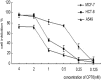
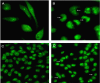
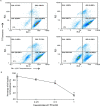
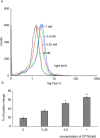
Camptothecin-20(s)-O-(2-pyrazolyl-1)acetic ester (CPT6) is a novel semi-synthetic analog of camptothecin. In a previous report, CPT6 possessed higher cytotoxic activity in vitro towards human breast tumor MCF-7 cells than topotecan. In this study, the antitumor activity of CPT6 on the human breast tumor MCF-7 cell line was analyzed using the MTT method. The underlying mechanism of CPT6 action was investigated by analyzing the cell cycle distribution, apoptotic proportion, changes in mitochondrial membrane potential, and intracellular Ca2+ concentration using flow cytometry. Nuclear and mitochondrial morphologies were also observed by laser scanning confocal and transmission electron microscopy. DNA damage was observed in MCF-7 cells treated with CPT6. Low-dose CPT6 had a significant cytotoxic effect and could inhibit proliferation and induce apoptosis in MCF-7 cells, possibly through cell nucleus fragmentation and DNA damage. CPT6 thus appears to display potent antitumor activity against human breast tumor MCF-7 cells via the induction of apoptosis, and may be a useful alternative drug for breast cancer therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Anti-proliferative and pro-apoptotic effect of CPT13, a novel camptothecin analog, on human colon cancer HCT8 cell line.Chem Biol Interact. 2008 Nov 25;176(2-3):165-72. doi: 10.1016/j.cbi.2008.07.005. Epub 2008 Jul 30. Chem Biol Interact. 2008. PMID: 18708040
-
Camptothecin-20(s)-O-[N-(3'α,12'α-dihydroxy-24'-carbonyl-5'β-cholan)]-lysine, a novel camptothecin analogue, induces apoptosis towards hepatocellular carcinoma SMMC-7721 cells.Molecules. 2011 Sep 13;16(9):7803-14. doi: 10.3390/molecules16097803. Molecules. 2011. PMID: 22143544 Free PMC article.
-
Apoptosis induction and cell cycle perturbation in human hepatoma hep G2 cells by 10-hydroxycamptothecin.Anticancer Drugs. 1999 Jul;10(6):569-76. doi: 10.1097/00001813-199907000-00009. Anticancer Drugs. 1999. PMID: 10885905
-
The novel camptothecin derivative, CPT211, induces cell cycle arrest and apoptosis in models of human breast cancer.Biomed Pharmacother. 2020 Aug;128:110309. doi: 10.1016/j.biopha.2020.110309. Epub 2020 Jun 4. Biomed Pharmacother. 2020. PMID: 32505820
-
Berberine induces apoptosis in breast cancer cells (MCF-7) through mitochondrial-dependent pathway.Eur J Pharmacol. 2010 Oct 25;645(1-3):70-8. doi: 10.1016/j.ejphar.2010.07.037. Epub 2010 Aug 4. Eur J Pharmacol. 2010. PMID: 20691179
Cited by
-
Generation Dependent Effects and Entrance to Mitochondria of Hybrid Dendrimers on Normal and Cancer Neuronal Cells In Vitro.Biomolecules. 2020 Mar 9;10(3):427. doi: 10.3390/biom10030427. Biomolecules. 2020. PMID: 32182909 Free PMC article.
-
The Influence of Graphene Oxide-Fe3O4 Differently Conjugated with 10-Hydroxycampthotecin and a Rotating Magnetic Field on Adenocarcinoma Cells.Int J Mol Sci. 2024 Jan 11;25(2):930. doi: 10.3390/ijms25020930. Int J Mol Sci. 2024. PMID: 38256006 Free PMC article.
-
Combined treatment with niclosamide and camptothecin enhances anticancer effect in U87 MG human glioblastoma cells.Oncotarget. 2022 May 5;13:642-658. doi: 10.18632/oncotarget.28227. eCollection 2022. Oncotarget. 2022. PMID: 35548329 Free PMC article.
-
Ruthenium Dendrimers against Human Lymphoblastic Leukemia 1301 Cells.Int J Mol Sci. 2020 Jun 9;21(11):4119. doi: 10.3390/ijms21114119. Int J Mol Sci. 2020. PMID: 32526993 Free PMC article.
-
Chemical Constituents of Eupatorium japonicum and Anti-Inflammatory, Cytotoxic, and Apoptotic Activities of Eupatoriopicrin on Cancer Stem Cells.Evid Based Complement Alternat Med. 2021 May 18;2021:6610347. doi: 10.1155/2021/6610347. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34055014 Free PMC article.
References
-
- Verschraegen C.F., Jaeckle K. Alternative administration of camptothecin analogues. Ann. N.Y. Acad. Sci. 2000;922:237–246. - PubMed
-
- Giovanell B.C., Stehlin J.S. DNA-topoisomerase I-targeted chemotherapy of human colon cancer in xenografts. Science. 1989;246:1046–1048. - PubMed
-
- Hsiang Y.H., Hertzberg R. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985;260:14873–14878. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

