Essential role for autophagy in the maintenance of immunological memory against influenza infection
- PMID: 24747745
- PMCID: PMC4066663
- DOI: 10.1038/nm.3521
Essential role for autophagy in the maintenance of immunological memory against influenza infection
Abstract
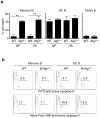
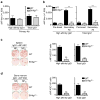
Vaccination has been the most widely used strategy to protect against viral infections for centuries. However, the molecular mechanisms governing the long-term persistence of immunological memory in response to vaccines remain unclear. Here we show that autophagy has a critical role in the maintenance of memory B cells that protect against influenza virus infection. Memory B cells displayed elevated levels of basal autophagy with increased expression of genes that regulate autophagy initiation or autophagosome maturation. Mice with B cell-specific deletion of Atg7 (B/Atg7(-/-) mice) showed normal primary antibody responses after immunization against influenza but failed to generate protective secondary antibody responses when challenged with influenza viruses, resulting in high viral loads, widespread lung destruction and increased fatality. Our results suggest that autophagy is essential for the survival of virus-specific memory B cells in mice and the maintenance of protective antibody responses required to combat infections.
Figures
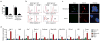

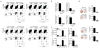
References
-
- Lenardo M, et al. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. - PubMed
-
- Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. - PubMed
-
- Kalia V, Sarkar S, Gourley TS, Rouse BT, Ahmed R. Differentiation of memory B and T cells. Curr Opin Immunol. 2006;18:255–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

