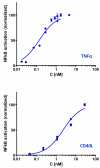
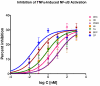
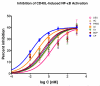
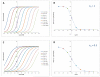
Effects of representative glucocorticoids on TNFα- and CD40L-induced NF-κB activation in sensor cells
- PMID: 24747770
- PMCID: PMC4049353
- DOI: 10.1016/j.steroids.2014.04.003
Effects of representative glucocorticoids on TNFα- and CD40L-induced NF-κB activation in sensor cells
Abstract
Glucocorticoids are an important class of anti-inflammatory/immunosuppressive drugs. A crucial part of their anti-inflammatory action results from their ability to repress proinflammatory transcription factors such as nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) upon binding to the glucocorticoid receptor (GR). Accordingly, sensor cells quantifying their effect on inflammatory signal-induced NF-κB activation can provide useful information regarding their potencies as well as their transrepression abilities. Here, we report results obtained on their effect in suppressing both the TNFα- and the CD40L-induced activation of NF-κB in sensor cells that contain an NF-κB-inducible SEAP construct. In these cells, we confirmed concentration-dependent NF-κB activation for both TNFα and CD40L at low nanomolar concentrations (EC50). Glucocorticoids tested included hydrocortisone, prednisolone, dexamethasone, loteprednol etabonate, triamcinolone acetonide, beclomethasone dipropionate, and clobetasol propionate. They all caused significant, but only partial inhibition of these activations in concentration-dependent manners that could be well described by sigmoid response-functions. Despite the limitations of only partial maximum inhibitions, this cell-based assay could be used to quantitate the suppressing ability of glucocorticoids (transrepression potency) on the expression of proinflammatory transcription factors caused by two different cytokines in parallel both in a detailed, full dose-response format as well as in a simpler single-dose format. Whereas inhibitory potencies obtained in the TNF assay correlated well with consensus glucocorticoid potencies (receptor-binding affinities, Kd, RBA, at the GR) for all compounds, the non-halogenated steroids (hydrocortisone, prednisolone, and loteprednol etabonate) were about an order of magnitude more potent than expected in the CD40 assay in this system.
Keywords: CD154; Corticosteroids; Hill equation; TNF; Transrepression.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Transrepression and transactivation potencies of inhaled glucocorticoids.Pharmazie. 2008 Dec;63(12):893-8. Pharmazie. 2008. PMID: 19177906
-
Fluticasone propionate and mometasone furoate have equivalent transcriptional potencies.Clin Exp Allergy. 2003 Jul;33(7):895-901. doi: 10.1046/j.1365-2222.2003.01709.x. Clin Exp Allergy. 2003. PMID: 12859444
-
Dissociated glucocorticoids with anti-inflammatory potential repress interleukin-6 gene expression by a nuclear factor-kappaB-dependent mechanism.Mol Pharmacol. 1999 Oct;56(4):797-806. Mol Pharmacol. 1999. PMID: 10496964
-
Mechanisms of glucocorticoid receptor signaling during inflammation.Mech Ageing Dev. 2004 Oct-Nov;125(10-11):697-706. doi: 10.1016/j.mad.2004.06.010. Mech Ageing Dev. 2004. PMID: 15541765 Review.
-
Cross-talk between pro-inflammatory transcription factors and glucocorticoids.Immunol Cell Biol. 2001 Aug;79(4):376-84. doi: 10.1046/j.1440-1711.2001.01025.x. Immunol Cell Biol. 2001. PMID: 11488985 Review.
Cited by
-
Endogenous circadian regulation of pro-inflammatory cytokines and chemokines in the presence of bacterial lipopolysaccharide in humans.Brain Behav Immun. 2015 Jul;47:4-13. doi: 10.1016/j.bbi.2014.11.003. Epub 2014 Nov 13. Brain Behav Immun. 2015. PMID: 25452149 Free PMC article.
-
TNF superfamily protein-protein interactions: feasibility of small- molecule modulation.Curr Drug Targets. 2015;16(4):393-408. doi: 10.2174/1389450116666150223115628. Curr Drug Targets. 2015. PMID: 25706111 Free PMC article. Review.
-
Aldo-Keto Reductases: Multifunctional Proteins as Therapeutic Targets in Diabetes and Inflammatory Disease.Adv Exp Med Biol. 2018;1032:173-202. doi: 10.1007/978-3-319-98788-0_13. Adv Exp Med Biol. 2018. PMID: 30362099 Free PMC article. Review.
-
Small-Molecule Inhibitors of the CD40-CD40L Costimulatory Protein-Protein Interaction.J Med Chem. 2017 Nov 9;60(21):8906-8922. doi: 10.1021/acs.jmedchem.7b01154. Epub 2017 Oct 25. J Med Chem. 2017. PMID: 29024591 Free PMC article.
-
Design, Synthesis, and Evaluation of Novel Immunomodulatory Small Molecules Targeting the CD40⁻CD154 Costimulatory Protein-Protein Interaction.Molecules. 2018 May 11;23(5):1153. doi: 10.3390/molecules23051153. Molecules. 2018. PMID: 29751636 Free PMC article.
References
-
- Schimmer BP, Parker KL. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Hardman JG, Limbird LE, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. McGraw-Hill; New York: 1996. pp. 1459–85.
-
- Barnes PJ. Therapeutic strategies for allergic diseases. Nature. 1999;402(Suppl.):B31–B8. - PubMed
-
- Avery MA, Woolfrey JR. Anti-inflammatory steroids. In: Abraham DJ, editor. Burger’s Medicinal Chemistry and Drug Discovery Vol 3, Cardiovascular Agents and Endocrines. 6th Edition Wiley; Hoboken, NJ: 2003. pp. 747–853.
-
- Bodor N, Buchwald P. Corticosteroid design for the treatment of asthma: structural insights and the therapeutic potential of soft corticosteroids. Curr Pharm Des. 2006;12:3241–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

