Deciphering the effect of the different N-glycosylation sites on the secretion, activity, and stability of cellobiohydrolase I from Trichoderma reesei
- PMID: 24747898
- PMCID: PMC4054209
- DOI: 10.1128/AEM.00261-14
Deciphering the effect of the different N-glycosylation sites on the secretion, activity, and stability of cellobiohydrolase I from Trichoderma reesei
Abstract
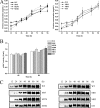
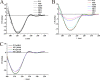
N-linked glycosylation modulates and diversifies the structures and functions of the eukaryotic proteome through both intrinsic and extrinsic effects on proteins. We investigated the significance of the three N-linked glycans on the catalytic domain of cellobiohydrolase I (CBH1) from the filamentous fungus Trichoderma reesei in its secretion and activity. While the removal of one or two N-glycosylation sites hardly affected the extracellular secretion of CBH1, eliminating all of the glycosylation sites did induce expression of the unfolded protein response (UPR) target genes, and secretion of this CBH1 variant was severely compromised in a calnexin gene deletion strain. Further characterization of the purified CBH1 variants showed that, compared to Asn270, the thermal reactivity of CBH1 was significantly decreased by removal of either Asn45 or Asn384 glycosylation site during the catalyzed hydrolysis of soluble substrate. Combinatorial loss of these two N-linked glycans further exacerbated the temperature-dependent inactivation. In contrast, this thermal labile property was less severe when hydrolyzing insoluble cellulose. Analysis of the structural integrity of CBH1 variants revealed that removal of N-glycosylation at Asn384 had a more pronounced effect on the integrity of regular secondary structure compared to the loss of Asn45 or Asn270. These data implicate differential roles of N-glycosylation modifications in contributing to the stability of specific functional regions of CBH1 and highlight the potential of improving the thermostability of CBH1 by tuning proper interactions between glycans and functional residues.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
The diversity of glycosylation of cellobiohydrolase I from Trichoderma reesei determined with mass spectrometry.Biochem Biophys Res Commun. 2019 Jan 15;508(3):818-824. doi: 10.1016/j.bbrc.2018.12.013. Epub 2018 Dec 7. Biochem Biophys Res Commun. 2019. PMID: 30528732
-
Substrate affinity and catalytic efficiency are improved by decreasing glycosylation sites in Trichoderma reesei cellobiohydrolase I expressed in Pichia pastoris.Biotechnol Lett. 2016 Mar;38(3):483-8. doi: 10.1007/s10529-015-1997-8. Epub 2015 Nov 23. Biotechnol Lett. 2016. PMID: 26597709
-
Impact of alg3 gene deletion on growth, development, pigment production, protein secretion, and functions of recombinant Trichoderma reesei cellobiohydrolases in Aspergillus niger.Fungal Genet Biol. 2013 Dec;61:120-32. doi: 10.1016/j.fgb.2013.09.004. Epub 2013 Sep 25. Fungal Genet Biol. 2013. PMID: 24076077
-
Novel cellulase profile of Trichoderma reesei strains constructed by cbh1 gene replacement with eg3 gene expression cassette.Acta Biochim Biophys Sin (Shanghai). 2004 Oct;36(10):667-72. doi: 10.1093/abbs/36.10.667. Acta Biochim Biophys Sin (Shanghai). 2004. PMID: 15483746
-
Enhancing the production of a heterologous Trametes laccase (LacA) by replacement of the major cellulase CBH1 in Trichoderma reesei.J Ind Microbiol Biotechnol. 2023 Feb 17;50(1):kuad002. doi: 10.1093/jimb/kuad002. J Ind Microbiol Biotechnol. 2023. PMID: 36690343 Free PMC article.
Cited by
-
From induction to secretion: a complicated route for cellulase production in Trichoderma reesei.Bioresour Bioprocess. 2021 Oct 22;8(1):107. doi: 10.1186/s40643-021-00461-8. Bioresour Bioprocess. 2021. PMID: 38650205 Free PMC article. Review.
-
In-depth characterization of Trichoderma reesei cellobiohydrolase TrCel7A produced in Nicotiana benthamiana reveals limitations of cellulase production in plants by host-specific post-translational modifications.Plant Biotechnol J. 2020 Mar;18(3):631-643. doi: 10.1111/pbi.13227. Epub 2019 Aug 17. Plant Biotechnol J. 2020. PMID: 31373133 Free PMC article.
-
Engineering the residual side chains of HAP phytases to improve their pepsin resistance and catalytic efficiency.Sci Rep. 2017 Feb 10;7:42133. doi: 10.1038/srep42133. Sci Rep. 2017. PMID: 28186144 Free PMC article.
-
Role of Protein Glycosylation in Host-Pathogen Interaction.Cells. 2020 Apr 20;9(4):1022. doi: 10.3390/cells9041022. Cells. 2020. PMID: 32326128 Free PMC article. Review.
-
Ugd Is Involved in the Synthesis of Glycans of Glycoprotein and LPS and Is Important for Cellulose Degradation in Cytophaga hutchinsonii.Microorganisms. 2025 Feb 11;13(2):395. doi: 10.3390/microorganisms13020395. Microorganisms. 2025. PMID: 40005761 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

