The influence of body position on cerebrospinal fluid pressure gradient and movement in cats with normal and impaired craniospinal communication
- PMID: 24748150
- PMCID: PMC3991613
- DOI: 10.1371/journal.pone.0095229
The influence of body position on cerebrospinal fluid pressure gradient and movement in cats with normal and impaired craniospinal communication
Abstract
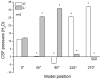
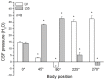
Intracranial hypertension is a severe therapeutic problem, as there is insufficient knowledge about the physiology of cerebrospinal fluid (CSF) pressure. In this paper a new CSF pressure regulation hypothesis is proposed. According to this hypothesis, the CSF pressure depends on the laws of fluid mechanics and on the anatomical characteristics inside the cranial and spinal space, and not, as is today generally believed, on CSF secretion, circulation and absorption. The volume and pressure changes in the newly developed CSF model, which by its anatomical dimensions and basic biophysical features imitates the craniospinal system in cats, are compared to those obtained on cats with and without the blockade of craniospinal communication in different body positions. During verticalization, a long-lasting occurrence of negative CSF pressure inside the cranium in animals with normal cranio-spinal communication was observed. CSF pressure gradients change depending on the body position, but those gradients do not enable unidirectional CSF circulation from the hypothetical site of secretion to the site of absorption in any of them. Thus, our results indicate the existence of new physiological/pathophysiological correlations between intracranial fluids, which opens up the possibility of new therapeutic approaches to intracranial hypertension.
Conflict of interest statement
Figures
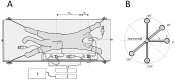
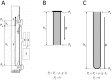
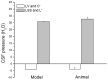
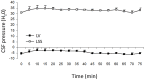
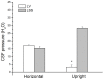
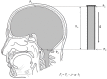
 , File S1). Thus, according to the law of fluid mechanics, inside that kind of space negative pressure appears without the changes of the fluid volume. According to the mentioned law, CSF inside the cranium should undergo the same fate (File S1). Namely, according to this law, negative value of the hydrostatic CSF pressure inside the cranium does not depend on the shape of the volume (File S1), but only on the distance between the point of measurement and foramen magnum (hc).
, File S1). Thus, according to the law of fluid mechanics, inside that kind of space negative pressure appears without the changes of the fluid volume. According to the mentioned law, CSF inside the cranium should undergo the same fate (File S1). Namely, according to this law, negative value of the hydrostatic CSF pressure inside the cranium does not depend on the shape of the volume (File S1), but only on the distance between the point of measurement and foramen magnum (hc).References
-
- Davson H, Welch K, Segal MB (1987) Physiology and pathophysiology of the cerebrospinal fluid. Edinburgh: Churchill-Livingstone. 1013 p.
-
- Sakka L, Coll G, Chazal J (2011) Anatomy and physiology of cerebrospinal fluid. Europ Ann Otorhinolaryngology, Head Neck Diseases 128: 309–316. - PubMed
-
- Dandy WE (1929) Where is cerebrospinal fluid absorbed? JAMA 92: 2012–2014.
-
- Brierly JF, Field EJ (1948) The connections of the cerebrospinal fluid space with the lymphatic system. J Anat 82: 153–166. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

