Similar metabolic changes induced by HIPVs exposure as herbivore in Ammopiptanthus mongolicus
- PMID: 24748156
- PMCID: PMC3991656
- DOI: 10.1371/journal.pone.0095474
Similar metabolic changes induced by HIPVs exposure as herbivore in Ammopiptanthus mongolicus
Abstract
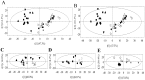
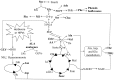
Herbivore-induced plant volatiles (HIPVs) are important compounds to prim neighboring undamaged plants; however, the mechanism for this priming process remains unclear. To reveal metabolic changes in plants exposed to HIPVs, metabolism of leaves and roots of Ammopiptanthus mongolicus seedlings exposed to HIPVs released from conspecific plants infested with larvae of Orgyia ericae were analyzed together with control and infested seedlings using nuclear magnetic resonance (NMR)-based metabolic technology and multi variate data analysis. Results presented showed that HIPVs exposure led to similar but specific metabolic changes compared with those induced by infestation in both leaves and roots. Furthermore, both HIPVs exposure and herbivore attack resulted in metabolic changes involving a series of primary and secondary metabolites in both leaves and roots. Taken together, these results suggested that priming of yet-damaged plants may be achieved by reconfiguring metabolic pathways in leaves and roots to make similar concentrations for all metabolites as those in seedlings infested. Therefore, we propose that improved readiness of defense induction of primed plants toward subsequent herbivore attack may be based on the similar metabolic profiling induced by HIPVs exposure as those caused by herbivore.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

