HIV-1 Nef inhibits Protease activity and its absence alters protein content of mature viral particles
- PMID: 24748174
- PMCID: PMC3991643
- DOI: 10.1371/journal.pone.0095352
HIV-1 Nef inhibits Protease activity and its absence alters protein content of mature viral particles
Abstract
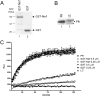
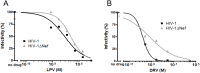
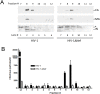
Nef is an important player for viral infectivity and AIDS progression, but the mechanisms involved are not completely understood. It was previously demonstrated that Nef interacts with GagPol through p6*-Protease region. Because p6* and Protease are involved in processing, we explored the effect of Nef on viral Protease activity and virion assembly. Using in vitro assays, we observed that Nef is highly capable of inhibiting Protease activity. The IC50 for nef-deficient viruses in drug susceptibility assays were 1.7- to 3.5-fold higher than the wild-type counterpart varying with the type of the Protease inhibitor used. Indicating that, in the absence of Nef, Protease is less sensitive to Protease inhibitors. We compared the protein content between wild-type and nef-deficient mature viral particles by gradient sedimentation and observed up to 2.7-fold reduction in the Integrase levels in nef-deficient mature particles. This difference in levels of Integrase correlated with the difference in infectivity levels of wild type and nef-deficient viral progeny. In addition, an overall decrease in the production of mature particles was detected in nef-deficient viruses. Collectively, our data support the hypothesis that the decreased infectivity typical of nef-deficient viruses is due to an abnormal function of the viral Protease, which is in turn associated with less mature particles being produced and the loss of Integrase content in these particles, and these results may characterize Nef as a regulator of viral Protease activity.
Conflict of interest statement
Figures


Similar articles
-
The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity.J Virol. 1998 Apr;72(4):3178-84. doi: 10.1128/JVI.72.4.3178-3184.1998. J Virol. 1998. PMID: 9525644 Free PMC article.
-
Interactions of processed Nef (58-206) with virion proteins of HIV type 1.AIDS Res Hum Retroviruses. 2004 Apr;20(4):399-407. doi: 10.1089/088922204323048140. AIDS Res Hum Retroviruses. 2004. PMID: 15157358
-
Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein.J Virol. 1996 Jul;70(7):4283-90. doi: 10.1128/JVI.70.7.4283-4290.1996. J Virol. 1996. PMID: 8676450 Free PMC article.
-
The Nef-infectivity enigma: mechanisms of enhanced lentiviral infection.Curr HIV Res. 2011 Oct;9(7):474-89. doi: 10.2174/157016211798842099. Curr HIV Res. 2011. PMID: 22103831 Free PMC article. Review.
-
Cell-dependent functional roles of HIV-1 Nef for virus replication (review).Int J Mol Med. 1999 Apr;3(4):427-30. doi: 10.3892/ijmm.3.4.427. Int J Mol Med. 1999. PMID: 10085418 Review.
Cited by
-
HIV-1 Nef Targets HDAC6 to Assure Viral Production and Virus Infection.Front Microbiol. 2019 Oct 30;10:2437. doi: 10.3389/fmicb.2019.02437. eCollection 2019. Front Microbiol. 2019. PMID: 31736889 Free PMC article.
-
Effect of transcription inhibition and generation of suppressive viral non-coding RNAs.Retrovirology. 2019 Apr 29;16(1):13. doi: 10.1186/s12977-019-0475-0. Retrovirology. 2019. PMID: 31036006 Free PMC article.
-
A Truncated Nef Peptide from SIVcpz Inhibits the Production of HIV-1 Infectious Progeny.Viruses. 2016 Jul 7;8(7):189. doi: 10.3390/v8070189. Viruses. 2016. PMID: 27399760 Free PMC article.
-
Structural Analysis of Retrovirus Assembly and Maturation.Viruses. 2021 Dec 29;14(1):54. doi: 10.3390/v14010054. Viruses. 2021. PMID: 35062258 Free PMC article. Review.
-
HIV Genome-Wide Protein Associations: a Review of 30 Years of Research.Microbiol Mol Biol Rev. 2016 Jun 29;80(3):679-731. doi: 10.1128/MMBR.00065-15. Print 2016 Sep. Microbiol Mol Biol Rev. 2016. PMID: 27357278 Free PMC article.
References
-
- Fackler OT, Moris A, Tibroni N, Giese SI, Glass B, et al. (2006) Functional characterization of HIV-1 Nef mutants in the context of viral infection. Virology 351: 322–339. - PubMed
-
- Garcia JV, Miller AD (1991) Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350: 508–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

