A tiered analytical approach for investigating poor quality emergency contraceptives
- PMID: 24748219
- PMCID: PMC3991657
- DOI: 10.1371/journal.pone.0095353
A tiered analytical approach for investigating poor quality emergency contraceptives
Abstract
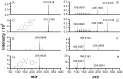
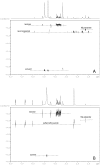
Reproductive health has been deleteriously affected by poor quality medicines. Emergency contraceptive pills (ECPs) are an important birth control method that women can use after unprotected coitus for reducing the risk of pregnancy. In response to the detection of poor quality ECPs commercially available in the Peruvian market we developed a tiered multi-platform analytical strategy. In a survey to assess ECP medicine quality in Peru, 7 out of 25 different batches showed inadequate release of levonorgestrel by dissolution testing or improper amounts of active ingredient. One batch was found to contain a wrong active ingredient, with no detectable levonorgestrel. By combining ultrahigh performance liquid chromatography-ion mobility spectrometry-mass spectrometry (UHPLC-IMS-MS) and direct analysis in real time MS (DART-MS) the unknown compound was identified as the antibiotic sulfamethoxazole. Quantitation by UHPLC-triple quadrupole tandem MS (QqQ-MS/MS) indicated that the wrong ingredient was present in the ECP sample at levels which could have significant physiological effects. Further chemical characterization of the poor quality ECP samples included the identification of the excipients by 2D Diffusion-Ordered Nuclear Magnetic Resonance Spectroscopy (DOSY 1H NMR) indicating the presence of lactose and magnesium stearate.
Conflict of interest statement
Figures



Similar articles
-
Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges.J Pharm Biomed Anal. 2014 Jan;87:176-90. doi: 10.1016/j.jpba.2013.04.037. Epub 2013 May 6. J Pharm Biomed Anal. 2014. PMID: 23721687 Review.
-
Ultra high performance liquid chromatography-time-of-flight high resolution mass spectrometry in the analysis of hexabromocyclododecane diastereomers: method development and comparative evaluation versus ultra high performance liquid chromatography coupled to Orbitrap high resolution mass spectrometry and triple quadrupole tandem mass spectrometry.J Chromatogr A. 2014 Oct 31;1366:73-83. doi: 10.1016/j.chroma.2014.09.021. Epub 2014 Sep 21. J Chromatogr A. 2014. PMID: 25262032
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
-
Over-the-counter access to levonorgestrel emergency contraception in South Texas: Does Over-the-counter mean ready to buy?Contraception. 2021 Sep;104(3):271-274. doi: 10.1016/j.contraception.2021.05.005. Epub 2021 May 23. Contraception. 2021. PMID: 34029556
-
Emergency contraceptive use among 5677 women seeking abortion in Shanghai, China.Hum Reprod. 2009 Jul;24(7):1612-8. doi: 10.1093/humrep/dep064. Epub 2009 Apr 9. Hum Reprod. 2009. PMID: 19359336
Cited by
-
Detecting falsified oral contraceptives by visual assessment and diffuse reflectance spectroscopy (350-2500 nm): the need for supplementing traditional pharmacopeia techniques and the public health implications.Heliyon. 2022 Oct 1;8(10):e10837. doi: 10.1016/j.heliyon.2022.e10837. eCollection 2022 Oct. Heliyon. 2022. PMID: 36217469 Free PMC article.
-
Poor quality male latex condoms found in Dominican Republic: Quality assurance evaluation and public health impact.PLoS One. 2019 Jan 7;14(1):e0210150. doi: 10.1371/journal.pone.0210150. eCollection 2019. PLoS One. 2019. PMID: 30615647 Free PMC article.
-
Triboelectric Nanogenerator (TENG) Mass Spectrometry of Falsified Antimalarials.Rapid Commun Mass Spectrom. 2018 Jun 23;32(18):1585-90. doi: 10.1002/rcm.8207. Online ahead of print. Rapid Commun Mass Spectrom. 2018. PMID: 29935091 Free PMC article.
-
"Scentsor": A Whole-Cell Yeast Biosensor with an Olfactory Reporter for Low-Cost and Equipment-Free Detection of Pharmaceuticals.ACS Sens. 2020 Oct 23;5(10):3025-3030. doi: 10.1021/acssensors.0c01344. Epub 2020 Oct 12. ACS Sens. 2020. PMID: 32964706 Free PMC article.
References
-
- Newton P, Fernández F, Green M, Primo-Carpenter J, White N (2009) Counterfeit And Substandard Anti-Infectives In Developing Countries; Sosa A, Byarugaba D, Amábile-Cuevas C, Hsueh P-R, Kariuki S, et al., editors: Springer.
-
- Nishtar S (2012) Pakistan's deadly cocktail of substandard drugs. Lancet 379: 1084–1085. - PubMed
-
- Dorlo TPC, Ravinetto RM, Beijnen JH, Boelaert M (2012) Commentary: Substandard medicines are the priority for neglected tropical diseases. Br Med J 345: e7518. - PubMed
-
- Smith RM, Schaefer MK, Kainer MA, Wise M, Finks J, et al.. (2012) Fungal Infections Associated with Contaminated Methylprednisolone Injections — Preliminary Report. N Engl J Med 0.. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

