Targeting c-Met receptor overcomes TRAIL-resistance in brain tumors
- PMID: 24748276
- PMCID: PMC3991662
- DOI: 10.1371/journal.pone.0095490
Targeting c-Met receptor overcomes TRAIL-resistance in brain tumors
Abstract
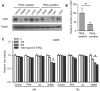
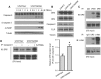
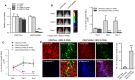
Tumor necrosis factor related apoptosis-inducing ligand (TRAIL) induced apoptosis specifically in tumor cells. However, with approximately half of all known tumor lines being resistant to TRAIL, the identification of TRAIL sensitizers and their mechanism of action become critical to broadly use TRAIL as a therapeutic agent. In this study, we explored whether c-Met protein contributes to TRAIL sensitivity. We found a direct correlation between the c-Met expression level and TRAIL resistance. We show that the knock down c-Met protein, but not inhibition, sensitized brain tumor cells to TRAIL-mediated apoptosis by interrupting the interaction between c-Met and TRAIL cognate death receptor (DR) 5. This interruption greatly induces the formation of death-inducing signaling complex (DISC) and subsequent downstream apoptosis signaling. Using intracranially implanted brain tumor cells and stem cell (SC) lines engineered with different combinations of fluorescent and bioluminescent proteins, we show that SC expressing a potent and secretable TRAIL (S-TRAIL) have a significant anti-tumor effect in mice bearing c-Met knock down of TRAIL-resistant brain tumors. To our best knowledge, this is the first study that demonstrates c-Met contributes to TRAIL sensitivity of brain tumor cells and has implications for developing effective therapies for brain tumor patients.
Conflict of interest statement
Figures

References
-
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, et al. (1991) Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. . Science 251: 802–804. - PubMed
-
- Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, et al. (1984) Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature 311: 29–33. - PubMed
-
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003) Met, metastasis, motility and more. . Nat Rev Mol Cell Biol 4: 915–925. - PubMed
-
- Abounader R, Lal B, Luddy C, Koe G, Davidson B, et al. (2002) In vivo targeting of SF/HGF and c-met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis. Faseb J 16: 108–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

