Microenvironmentally controlled secondary structure motifs of apolipoprotein A-I derived peptides
- PMID: 24748322
- PMCID: PMC4067536
- DOI: 10.1007/s11010-014-2050-2
Microenvironmentally controlled secondary structure motifs of apolipoprotein A-I derived peptides
Abstract
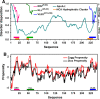
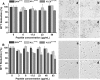
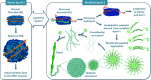
The structure of apolipoprotein A-I (apoA-I), the major protein of HDL, has been extensively studied in past years. Nevertheless, its corresponding three-dimensional structure has been difficult to obtain due to the frequent conformational changes observed depending on the microenvironment. Although the function of each helical segment of this protein remains unclear, it has been observed that the apoA-I amino (N) and carboxy-end (C) domains are directly involved in receptor-recognition, processes that determine the diameter for HDL particles. In addition, it has been observed that the high structural plasticity of these segments might be related to several amyloidogenic processes. In this work, we studied a series of peptides derived from the N- and C-terminal domains representing the most hydrophobic segments of apoA-I. Measurements carried out using circular dichroism in all tested peptides evidenced that the lipid environment promotes the formation of α-helical structures, whereas an aqueous environment facilitates a strong tendency to adopt β-sheet/disordered conformations. Electron microscopy observations showed the formation of amyloid-like structures similar to those found in other well-defined amyloidogenic proteins. Interestingly, when the apoA-I peptides were incubated under conditions that promote stable globular structures, two of the peptides studied were cytotoxic to microglia and mouse macrophage cells. Our findings provide an insight into the physicochemical properties of key segments contained in apoA-I which may be implicated in disorder-to-order transitions that in turn maintain the delicate equilibrium between both, native and abnormal conformations, and therefore control its propensity to become involved in pathological processes.
Figures


Similar articles
-
Amyloidogenic Mutation Promotes Fibril Formation of the N-terminal Apolipoprotein A-I on Lipid Membranes.J Biol Chem. 2015 Aug 21;290(34):20947-20959. doi: 10.1074/jbc.M115.664227. Epub 2015 Jul 14. J Biol Chem. 2015. PMID: 26175149 Free PMC article.
-
Heparin and Methionine Oxidation Promote the Formation of Apolipoprotein A-I Amyloid Comprising α-Helical and β-Sheet Structures.Biochemistry. 2017 Mar 21;56(11):1632-1644. doi: 10.1021/acs.biochem.6b01120. Epub 2017 Mar 13. Biochemistry. 2017. PMID: 27992182
-
Deletion of single amino acid E235 affects the structure and lipid interaction of human apolipoprotein A-I C-terminal peptides.Chem Pharm Bull (Tokyo). 2009 May;57(5):499-503. doi: 10.1248/cpb.57.499. Chem Pharm Bull (Tokyo). 2009. PMID: 19420782
-
The crystal structure of the C-terminal truncated apolipoprotein A-I sheds new light on amyloid formation by the N-terminal fragment.Biochemistry. 2012 Jan 10;51(1):10-8. doi: 10.1021/bi2017014. Epub 2011 Dec 29. Biochemistry. 2012. PMID: 22229410 Free PMC article. Review.
-
[A turning point in the knowledge of the structure-function-activity relations of elastin].J Soc Biol. 2001;195(2):181-93. J Soc Biol. 2001. PMID: 11727705 Review. French.
Cited by
-
Order in Disorder as Observed by the "Hydrophobic Cluster Analysis" of Protein Sequences.Proteomics. 2018 Nov;18(21-22):e1800054. doi: 10.1002/pmic.201800054. Epub 2018 Oct 30. Proteomics. 2018. PMID: 30299594 Free PMC article. Review.
-
Amyloidogenic propensity of a natural variant of human apolipoprotein A-I: stability and interaction with ligands.PLoS One. 2015 May 7;10(5):e0124946. doi: 10.1371/journal.pone.0124946. eCollection 2015. PLoS One. 2015. PMID: 25950566 Free PMC article.
-
Amyloid-Forming Properties of Human Apolipoproteins: Sequence Analyses and Structural Insights.Adv Exp Med Biol. 2015;855:175-211. doi: 10.1007/978-3-319-17344-3_8. Adv Exp Med Biol. 2015. PMID: 26149931 Free PMC article. Review.
-
Impact of cholesterol-pathways on breast cancer development, a metabolic landscape.J Cancer. 2021 May 19;12(14):4307-4321. doi: 10.7150/jca.54637. eCollection 2021. J Cancer. 2021. PMID: 34093831 Free PMC article. Review.
-
Modulation of Amyloidogenesis Controlled by the C-Terminal Domain of Islet Amyloid Polypeptide Shows New Functions on Hepatocyte Cholesterol Metabolism.Front Endocrinol (Lausanne). 2018 Jun 25;9:331. doi: 10.3389/fendo.2018.00331. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29988450 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

