Improvements of vaginal atrophy without systemic side effects after topical application of Pueraria mirifica, a phytoestrogen-rich herb, in postmenopausal cynomolgus macaques
- PMID: 24748397
- PMCID: PMC4085389
- DOI: 10.1262/jrd.2013-144
Improvements of vaginal atrophy without systemic side effects after topical application of Pueraria mirifica, a phytoestrogen-rich herb, in postmenopausal cynomolgus macaques
Abstract
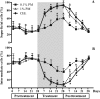
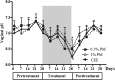
The estrogenic efficacy of topical vaginal application of Pueraria mirifica extract (PM) on the restoration of vaginal atrophy, and the presence of any systemic side effects, were investigated in postmenopausal cynomolgus macaques. Twelve postmenopausal cynomolgus macaques, with complete cessation of menstruation for at least 5 years before start of this experiment, were divided into three groups. They received a topical vaginal application daily of 0.1 or 1% (w/w) PM cream or a conjugated equine estrogen (CEE) cream (a mixture of estrone, equilin, 17β-dihydroequilin, 17α-estradiol and 17α-dihydroequilin at 0.625 mg total estrogen/g cream) for 28 days. Estrogenic efficacy was assessed weekly by vaginal cytology assay and vaginal pH measurement, whilst the plasma luteinizing hormone (LH) and sex skin coloration levels were determined at the end of each treatment period to evaluate the systemic side effects. PM significantly increased the proportion of superficial cells in a dose-dependent manner, with a similar efficacy between 1% (w/w) PM and CEE. Together with increased vaginal maturation, PM decreased the vaginal pH to acidic levels, as observed in the CEE group. PM induced no detected systemic side effects, whilst CEE decreased the plasma LH level and increased the reddish color of the sex skin during the posttreatment period. Topical vaginal treatment with PM stimulated the maturation of the vaginal epithelium without causing systemic side effects in postmenopausal monkeys. The implication is that PM could be a safer alternative to treat vaginal atrophy in postmenopausal women.
Figures


Similar articles
-
Comparison of Pueraria mirifica gel and conjugated equine estrogen cream effects on vaginal health in postmenopausal women.Menopause. 2017 Feb;24(2):210-215. doi: 10.1097/GME.0000000000000742. Menopause. 2017. PMID: 27749740 Clinical Trial.
-
Effect of Pueraria mirifica on vaginal health.Menopause. 2007 Sep-Oct;14(5):919-24. doi: 10.1097/gme.0b013e3180399486. Menopause. 2007. PMID: 17415017 Clinical Trial.
-
An overview of the phytoestrogen effect on vaginal health and dyspareunia in peri- and post-menopausal women.Post Reprod Health. 2019 Mar;25(1):11-20. doi: 10.1177/2053369118823365. Epub 2019 Feb 20. Post Reprod Health. 2019. PMID: 30786797 Review.
-
Using vaginal cytology to assess the estrogenic activity of phytoestrogen-rich herb.J Ethnopharmacol. 2006 Oct 11;107(3):354-60. doi: 10.1016/j.jep.2006.03.026. Epub 2006 Apr 6. J Ethnopharmacol. 2006. PMID: 16730147
-
Practical aspects in the management of vaginal atrophy and sexual dysfunction in perimenopausal and postmenopausal women.J Sex Med. 2005 Sep;2 Suppl 3:154-65. doi: 10.1111/j.1743-6109.2005.00131.x. J Sex Med. 2005. PMID: 16422792 Review.
Cited by
-
Dynamics of phytoestrogen, isoflavonoids, and its isolation from stems of Pueraria lobata (Willd.) Ohwi growing in Democratic People's Republic of Korea.J Food Drug Anal. 2015 Sep;23(3):538-544. doi: 10.1016/j.jfda.2015.04.003. Epub 2015 May 18. J Food Drug Anal. 2015. PMID: 28911713 Free PMC article.
-
Effect of fennel vaginal cream on sexual function in postmenopausal women: A double blind randomized controlled trial.J Med Life. 2018 Jan-Mar;11(1):24-28. J Med Life. 2018. PMID: 29696061 Free PMC article. Clinical Trial.
-
A prospective study of vaginal topical pretreatment of compound sea-buckthorn oil suppository in postmenopausal women prior to colposcopy.Sci Rep. 2025 Mar 26;15(1):10364. doi: 10.1038/s41598-025-95036-4. Sci Rep. 2025. PMID: 40133564 Free PMC article. Clinical Trial.
References
-
- Hextall A. Oestrogens and lower urinary tract function. Maturitas 2000; 36: 83–92 - PubMed
-
- Stenberg A, Heimer G, Ulmsten U, Cnattingius S. Prevalence of genitourinary and other climacteric symptoms in 61-year-old women. Maturitas 1996; 24: 31–36 - PubMed
-
- Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician 2000; 61: 3090–3096 - PubMed
-
- Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2006; 18: CD001500 - PubMed
-
- Lynch C. Vaginal estrogen therapy for the treatment of atrophic vaginitis. J Womens Health (Larchmt) 2009; 18: 1595–1606 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

