Hsp70 regulates the doxorubicin-mediated heart failure in Hsp70-transgenic mice
- PMID: 24748476
- PMCID: PMC4389845
- DOI: 10.1007/s12192-014-0509-4
Hsp70 regulates the doxorubicin-mediated heart failure in Hsp70-transgenic mice
Abstract
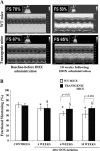
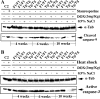
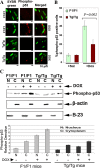
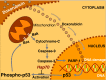
The aim of this study was to investigate the potential protective effect of the Hsp70 protein in the cardiac dysfunction induced by doxorubicin (DOX) and the mechanisms of its action. For this purpose, we used both wild-type mice (F1/F1) and Hsp70-transgenic mice (Tg/Tg) overexpressing human HSP70. Both types were subjected to chronic DOX administration (3 mg/kg intraperitoneally every week for 10 weeks, with an interval from weeks 4 to 6). Primary cell cultures isolated from embryos of these mice were also studied. During DOX administration, the mortality rate as well as weight reduction were lower in Tg/Tg compared to F1/F1 mice (P < 0.05). In vivo cardiac function assessment by transthoracic echocardiography showed that the reduction in left ventricular systolic function observed after DOX administration was lower in Tg/Tg mice (P < 0.05). The study in primary embryonic cell lines showed that the apoptosis after incubation with DOX was reduced in cells overexpressing Hsp70 (Tg/Tg), while the apoptotic pathway that was activated by DOX administration involved activated protein factors such as p53, Bax, caspase-9, caspase-3, and PARP-1. In myocardial protein extracts from identical mice with DOX-induced heart failure, the particular activated apoptotic pathway was confirmed, while the presence of Hsp70 appeared to inhibit the apoptotic pathway upstream of the p53 activation. Our results, in this DOX-induced heart failure model, indicate that Hsp70 overexpression in Tg/Tg transgenic mice provides protection from myocardial damage via an Hsp70-block in p53 activation, thus reducing the subsequent apoptotic mechanism.
Figures



Similar articles
-
The increased expression of the inducible Hsp70 (HSP70A1A) in serum of patients with heart failure and its protective effect against the cardiotoxic agent doxorubicin.Mol Cell Biochem. 2019 May;455(1-2):41-59. doi: 10.1007/s11010-018-3469-7. Epub 2018 Nov 2. Mol Cell Biochem. 2019. PMID: 30390173
-
Diacylglycerol Kinase ζ Attenuates Doxorubicin-Induced Cardiotoxicity Through p53 Degradation.J Am Heart Assoc. 2025 Jan 7;14(1):e035608. doi: 10.1161/JAHA.124.035608. Epub 2024 Dec 24. J Am Heart Assoc. 2025. PMID: 39719406 Free PMC article.
-
Serine mutations in overexpressed Hsp27 abrogate the protection against doxorubicin-induced p53-dependent cardiac apoptosis in mice.Am J Physiol Heart Circ Physiol. 2021 Nov 1;321(5):H963-H975. doi: 10.1152/ajpheart.00027.2020. Epub 2021 Sep 3. Am J Physiol Heart Circ Physiol. 2021. PMID: 34477462
-
Senescence marker protein 30 has a cardio-protective role in doxorubicin-induced cardiac dysfunction.PLoS One. 2013 Dec 31;8(12):e79093. doi: 10.1371/journal.pone.0079093. eCollection 2013. PLoS One. 2013. PMID: 24391705 Free PMC article.
-
Mitochondrial aldehyde dehydrogenase 2 plays protective roles in heart failure after myocardial infarction via suppression of the cytosolic JNK/p53 pathway in mice.J Am Heart Assoc. 2014 Sep 18;3(5):e000779. doi: 10.1161/JAHA.113.000779. J Am Heart Assoc. 2014. PMID: 25237043 Free PMC article.
Cited by
-
Toxic Effects of Methamphetamine on Perivascular Health: Co-morbid Effects of Stress and Alcohol Use Disorders.Curr Neuropharmacol. 2021;19(12):2092-2107. doi: 10.2174/1570159X19666210803150023. Curr Neuropharmacol. 2021. PMID: 34344290 Free PMC article. Review.
-
Lingguizhugan decoction attenuates doxorubicin-induced heart failure in rats by improving TT-SR microstructural remodeling.BMC Complement Altern Med. 2019 Dec 11;19(1):360. doi: 10.1186/s12906-019-2771-6. BMC Complement Altern Med. 2019. PMID: 31829159 Free PMC article.
-
The increased expression of the inducible Hsp70 (HSP70A1A) in serum of patients with heart failure and its protective effect against the cardiotoxic agent doxorubicin.Mol Cell Biochem. 2019 May;455(1-2):41-59. doi: 10.1007/s11010-018-3469-7. Epub 2018 Nov 2. Mol Cell Biochem. 2019. PMID: 30390173
-
Mitochondrial pathways to cardiac recovery: TFAM.Heart Fail Rev. 2016 Sep;21(5):499-517. doi: 10.1007/s10741-016-9561-8. Heart Fail Rev. 2016. PMID: 27166683 Free PMC article. Review.
-
Heat Shock Proteins: Connectors between Heart and Kidney.Cells. 2021 Jul 30;10(8):1939. doi: 10.3390/cells10081939. Cells. 2021. PMID: 34440708 Free PMC article. Review.
References
-
- Abe T, Fukamachi Y, Kanazava Y, Purukawa H, Shimizu K, Hirano T, Kasai H, Kashimura M, Higashi K. Inhibition of nucleolar function and morphological change by adriamycin associated with heat shock protein 70 accumulation. Jpn J Cancer Res. 1996;87:945–951. doi: 10.1111/j.1349-7006.1996.tb02124.x. - DOI - PMC - PubMed
-
- Achanta G, Pelicano H, Feng L, Plunkett W, Huang P. Interaction of p53 and DNA-PK in response to nucleoside analogues: potential role as a sensor complex for DNA damage. Cancer Res. 2001;61:8723–8729. - PubMed
-
- Adachi H, Katsuno M, Minamiyama M, Sang C, Pagoulatos G, Angelidis C, Kusakabe M, Yoshiki A, Kobayashi Y, Doyu M, Sobue G. Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein. J Neurosci. 2003;23:2203–2211. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

