CD8 T cells use IFN-γ to protect against the lethal effects of a respiratory poxvirus infection
- PMID: 24748494
- PMCID: PMC4036466
- DOI: 10.4049/jimmunol.1400256
CD8 T cells use IFN-γ to protect against the lethal effects of a respiratory poxvirus infection
Abstract
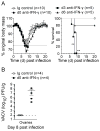
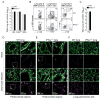
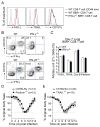
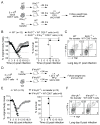
CD8 T cells are a key component of immunity to many viral infections. They achieve this through using an array of effector mechanisms, but precisely which component/s are required for protection against a respiratory orthopox virus infection remains unclear. Using a model of respiratory vaccinia virus infection in mice, we could specifically determine the relative contribution of perforin, TRAIL, and IFN-γ-mediated pathways in protection against virus induced morbidity and mortality. Unexpectedly, we observed that protection against death was mediated by IFN-γ without any involvement of the perforin or TRAIL-dependent pathways. IFN-γ mRNA and protein levels in the lung peaked between days 3 and 6 postinfection. This enhanced response coincided with the emergence of virus-specific CD8 T cells in the lung and the cessation of weight loss. Transfer experiments indicated that CD8 T cell-autonomous expression of IFN-γ restricts virus-induced lung pathology and dissemination to visceral tissues and is necessary for clearance of virus. Most significantly, we show that CD8 T cell-derived IFN-γ is sufficient to protect mice in the absence of CD4 and B-lymphocytes. Thus, our findings reveal a previously unappreciated mechanism by which effector CD8 T cells afford protection against a highly virulent respiratory orthopox virus infection.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures


References
-
- Moss B. Poxviridae: the viruses and their replication. In: Knipe DM, editor. Fields Virology. 5. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2905–2946.
-
- Arita I, Jezek Z, Khodakevich L, Ruti K. Human monkeypox: a newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. The American journal of tropical medicine and hygiene. 1985;34:781–789. - PubMed
-
- Baxby D, Bennett M, Getty B. Human cowpox 1969–93: a review based on 54 cases. The British journal of dermatology. 1994;131:598–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

