Sustained hyperoxia-induced NF-κB activation improves survival and preserves lung development in neonatal mice
- PMID: 24748603
- PMCID: PMC4060010
- DOI: 10.1152/ajplung.00001.2014
Sustained hyperoxia-induced NF-κB activation improves survival and preserves lung development in neonatal mice
Abstract
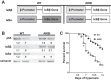
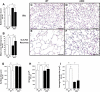
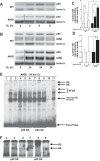
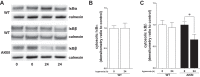
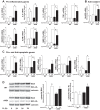
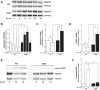
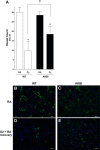
Oxygen toxicity contributes to the pathogenesis of bronchopulmonary dysplasia (BPD). Neonatal mice exposed to hyperoxia develop a simplified lung structure that resembles BPD. Sustained activation of the transcription factor NF-κB and increased expression of protective target genes attenuate hyperoxia-induced mortality in adults. However, the effect of enhancing hyperoxia-induced NF-κB activity on lung injury and development in neonatal animals is unknown. We performed this study to determine whether sustained NF-κB activation, mediated through IκBβ overexpression, preserves lung development in neonatal animals exposed to hyperoxia. Newborn wild-type (WT) and IκBβ-overexpressing (AKBI) mice were exposed to hyperoxia (>95%) or room air from day of life (DOL) 0-14, after which all animals were kept in room air. Survival curves were generated through DOL 14. Lung development was assessed using radial alveolar count (RAC) and mean linear intercept (MLI) at DOL 3 and 28 and pulmonary vessel density at DOL 28. Lung tissue was collected, and NF-κB activity was assessed using Western blot for IκB degradation and NF-κB nuclear translocation. WT mice demonstrated 80% mortality through 14 days of exposure. In contrast, AKBI mice demonstrated 60% survival. Decreased RAC, increased MLI, and pulmonary vessel density caused by hyperoxia in WT mice were significantly attenuated in AKBI mice. These findings were associated with early and sustained NF-κB activation and expression of cytoprotective target genes, including vascular endothelial growth factor receptor 2. We conclude that sustained hyperoxia-induced NF-κB activation improves neonatal survival and preserves lung development. Potentiating early NF-κB activity after hyperoxic exposure may represent a therapeutic intervention to prevent BPD.
Keywords: IκBβ hyperoxic lung injury; NF-κB; bronchopulmonary dysplasia; lung development.
Copyright © 2014 the American Physiological Society.
Figures

Similar articles
-
IκBβ-mediated NF-κB activation confers protection against hyperoxic lung injury.Am J Respir Cell Mol Biol. 2014 Feb;50(2):429-38. doi: 10.1165/rcmb.2013-0303OC. Am J Respir Cell Mol Biol. 2014. PMID: 24066808 Free PMC article.
-
Caffeine prevents hyperoxia-induced lung injury in neonatal mice through NLRP3 inflammasome and NF-κB pathway.Respir Res. 2020 Jun 8;21(1):140. doi: 10.1186/s12931-020-01403-2. Respir Res. 2020. PMID: 32513156 Free PMC article.
-
Adipose mesenchymal stem cells-derived exosomes attenuated hyperoxia-induced lung injury in neonatal rats via inhibiting the NF-κB signaling pathway.Pediatr Pulmonol. 2024 Oct;59(10):2523-2534. doi: 10.1002/ppul.27057. Epub 2024 May 21. Pediatr Pulmonol. 2024. PMID: 38771197
-
Manipulation of gene expression by oxygen: a primer from bedside to bench.Pediatr Res. 2009 Jul;66(1):3-10. doi: 10.1203/PDR.0b013e3181a2c184. Pediatr Res. 2009. PMID: 19287338 Free PMC article. Review.
-
Halogen exposure injury in the developing lung.Ann N Y Acad Sci. 2020 Nov;1480(1):30-43. doi: 10.1111/nyas.14445. Epub 2020 Aug 1. Ann N Y Acad Sci. 2020. PMID: 32738176 Free PMC article. Review.
Cited by
-
Oxygen toxicity: cellular mechanisms in normobaric hyperoxia.Cell Biol Toxicol. 2023 Feb;39(1):111-143. doi: 10.1007/s10565-022-09773-7. Epub 2022 Sep 16. Cell Biol Toxicol. 2023. PMID: 36112262 Free PMC article. Review.
-
Endotoxemia Induces IκBβ/NF-κB-Dependent Endothelin-1 Expression in Hepatic Macrophages.J Immunol. 2015 Oct 15;195(8):3866-79. doi: 10.4049/jimmunol.1501017. Epub 2015 Sep 4. J Immunol. 2015. PMID: 26342031 Free PMC article.
-
Looking ahead: where to next for animal models of bronchopulmonary dysplasia?Cell Tissue Res. 2017 Mar;367(3):457-468. doi: 10.1007/s00441-016-2534-3. Epub 2016 Dec 5. Cell Tissue Res. 2017. PMID: 27917436 Free PMC article. Review.
-
Bacterial Colonization within the First Six Weeks of Life and Pulmonary Outcome in Preterm Infants <1000 g.J Clin Med. 2020 Jul 15;9(7):2240. doi: 10.3390/jcm9072240. J Clin Med. 2020. PMID: 32679682 Free PMC article.
-
Intrauterine growth restriction decreases NF-κB signaling in fetal pulmonary artery endothelial cells of fetal sheep.Am J Physiol Lung Cell Mol Physiol. 2018 Sep 1;315(3):L348-L359. doi: 10.1152/ajplung.00052.2018. Epub 2018 May 3. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29722560 Free PMC article.
References
-
- Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 291: L119–L127, 2006 - PubMed
-
- Balasubramaniam V, Ryan SL, Seedorf GJ, Roth EV, Heumann TR, Yoder MC, Ingram DA, Hogan CJ, Markham NE, Abman SH. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol 298: L315–L323, 2010 - PMC - PubMed
-
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science 274: 782–784, 1996 - PubMed
-
- Ben-Ari J, Makhoul IR, Dorio RJ, Buckley S, Warburton D, Walker SM. Cytokine response during hyperoxia: sequential production of pulmonary tumor necrosis factor and interleukin-6 in neonatal rats. Isr Med Assoc J 2: 365–369, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

