Txc, a new type II secretion system of Pseudomonas aeruginosa strain PA7, is regulated by the TtsS/TtsR two-component system and directs specific secretion of the CbpE chitin-binding protein
- PMID: 24748613
- PMCID: PMC4054165
- DOI: 10.1128/JB.01563-14
Txc, a new type II secretion system of Pseudomonas aeruginosa strain PA7, is regulated by the TtsS/TtsR two-component system and directs specific secretion of the CbpE chitin-binding protein
Abstract
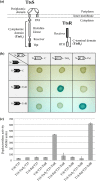
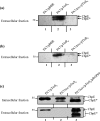
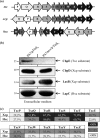
We present here the functional characterization of a third complete type II secretion system (T2SS) found in newly sequenced Pseudomonas aeruginosa strain PA7. We call this system Txc (third Xcp homolog). This system is encoded by the RGP69 region of genome plasticity found uniquely in strain PA7. In addition to the 11 txc genes, RGP69 contains two additional genes encoding a possible T2SS substrate and a predicted unorthodox sensor protein, TtsS (type II secretion sensor). We also identified a gene encoding a two-component response regulator called TtsR (type II secretion regulator), which is located upstream of the ttsS gene and just outside RGP69. We show that TtsS and TtsR constitute a new and functional two-component system that controls the production and secretion of the RGP69-encoded T2SS substrate in a Txc-dependent manner. Finally, we demonstrate that this Txc-secreted substrate binds chitin, and we therefore name it CbpE (chitin-binding protein E).
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

