Construction and characterization of a Borrelia burgdorferi strain with conditional expression of the essential telomere resolvase, ResT
- PMID: 24748617
- PMCID: PMC4054159
- DOI: 10.1128/JB.01435-13
Construction and characterization of a Borrelia burgdorferi strain with conditional expression of the essential telomere resolvase, ResT
Abstract
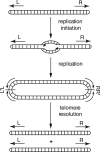
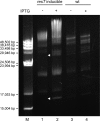
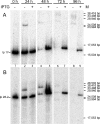
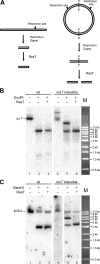
Borrelia species are unique in the bacterial world in possessing segmented genomes which sometimes contain over 20 genetic elements. Most elements are linear and contain covalently closed hairpin ends requiring a specialized process, telomere resolution, for their generation. Hairpin telomere resolution is mediated by the telomere resolvase, ResT. Although the process has been studied extensively in vitro, the essential nature of the resT gene has precluded biological studies to further probe the role of ResT. In this work, we have generated a B. burgdorferi strain that carries an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible resT gene controlled by a tightly regulated promoter. ResT is expressed in this strain at ~14,000 monomers per cell, similar to the ~15,000 monomers observed for the parental strain. We demonstrate ResT depletion with a half-life of 16 h upon IPTG washout. ResT depletion resulted in arrested growth 48 h after washout. Interestingly, not all spirochetes died after ResT washout, and at least 15% remained quiescent and could be resuscitated even at 2 weeks postwashout. Significant levels of DNA synthesis were not observed upon growth arrest, suggesting that ResT might interact directly or indirectly with factors controlling the initiation or elongation of DNA synthesis. Analysis of the linear plasmids lp17 and lp28-2 showed that the linear forms of these plasmids began to disappear and be replaced by higher-molecular-weight forms by 24 h post-IPTG washout. Treatment of DNA from the ResT-depleted strain with ResT in vitro revealed the presence of replicated telomeres expected in replication intermediates.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
The Borrelia burgdorferi telomere resolvase, ResT, possesses ATP-dependent DNA unwinding activity.Nucleic Acids Res. 2017 Feb 17;45(3):1319-1329. doi: 10.1093/nar/gkw1243. Nucleic Acids Res. 2017. PMID: 28180323 Free PMC article.
-
Characterization and in vitro reaction properties of 19 unique hairpin telomeres from the linear plasmids of the lyme disease spirochete.J Biol Chem. 2009 Mar 13;284(11):7264-72. doi: 10.1074/jbc.M808918200. Epub 2009 Jan 2. J Biol Chem. 2009. PMID: 19122193 Free PMC article.
-
Differential telomere processing by Borrelia telomere resolvases in vitro but not in vivo.J Bacteriol. 2006 Nov;188(21):7378-86. doi: 10.1128/JB.00760-06. Epub 2006 Aug 25. J Bacteriol. 2006. PMID: 16936037 Free PMC article.
-
Split target specificity of ResT: a design for protein delivery, site selectivity and regulation of enzyme activity?Mol Microbiol. 2007 May;64(3):575-9. doi: 10.1111/j.1365-2958.2007.05700.x. Mol Microbiol. 2007. PMID: 17462008 Review.
-
Hairpin telomeres and genome plasticity in Borrelia: all mixed up in the end.Mol Microbiol. 2005 Nov;58(3):625-35. doi: 10.1111/j.1365-2958.2005.04872.x. Mol Microbiol. 2005. PMID: 16238614 Review.
Cited by
-
Genetic Manipulation of Borrelia Spp.Curr Top Microbiol Immunol. 2018;415:113-140. doi: 10.1007/82_2017_51. Curr Top Microbiol Immunol. 2018. PMID: 28918538 Free PMC article. Review.
-
Genetic Manipulation of Borrelia.Curr Issues Mol Biol. 2021;42:307-332. doi: 10.21775/cimb.042.307. Epub 2020 Dec 10. Curr Issues Mol Biol. 2021. PMID: 33300496 Free PMC article. Review.
-
Combining short- and long-read sequencing unveils geographically structured diversity in Borrelia miyamotoi.iScience. 2024 Jul 30;27(9):110616. doi: 10.1016/j.isci.2024.110616. eCollection 2024 Sep 20. iScience. 2024. PMID: 39262806 Free PMC article.
-
The Borrelia burgdorferi telomere resolvase, ResT, possesses ATP-dependent DNA unwinding activity.Nucleic Acids Res. 2017 Feb 17;45(3):1319-1329. doi: 10.1093/nar/gkw1243. Nucleic Acids Res. 2017. PMID: 28180323 Free PMC article.
-
Single stranded DNA annealing is a conserved activity of telomere resolvases.PLoS One. 2021 Feb 4;16(2):e0246212. doi: 10.1371/journal.pone.0246212. eCollection 2021. PLoS One. 2021. PMID: 33539370 Free PMC article.
References
-
- Samuels DS, Radolf JD. 2010. Borrelia: molecular biology, host interaction and pathogenesis. Caister Academic Press, Norfolk, United Kingdom
-
- Dennis DT, Hayes EB. 2002. Epidemiology of Lyme borreliosis, p 251–280 In Gray JS, Kahl O, Lane RS, Stanek G. (ed), Lyme borreliosis: biology, epidemiology and control. CAB International, Wallingford, United Kingdom
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

