Towards modelling the flexible timing of shoot development: simulation of maize organogenesis based on coordination within and between phytomers
- PMID: 24748619
- PMCID: PMC4217678
- DOI: 10.1093/aob/mcu051
Towards modelling the flexible timing of shoot development: simulation of maize organogenesis based on coordination within and between phytomers
Abstract
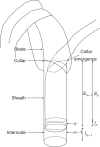
Background and aims: Experimental evidence challenges the approximation, central in crop models, that developmental events follow a fixed thermal time schedule, and indicates that leaf emergence events play a role in the timing of development. The objective of this study was to build a structural development model of maize (Zea mays) based on a set of coordination rules at organ level that regulate duration of elongation, and to show how the distribution of leaf sizes emerges from this.
Methods: A model of maize development was constructed based on three coordination rules between leaf emergence events and the dynamics of organ extension. The model was parameterized with data from maize grown at a low plant population density and tested using data from maize grown at high population density.
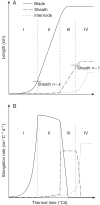
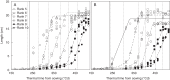
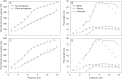
Key results: The model gave a good account of the timing and duration of organ extension. By using initial conditions associated with high population density, the model reproduced well the increase in blade elongation duration and the delay in sheath extension in high-density populations compared with low-density populations. Predictions of the sizes of sheaths at high density were accurate, whereas predictions of the dynamics of blade length were accurate up to rank 9; moderate overestimation of blade length occurred at higher ranks.
Conclusions: A set of simple rules for coordinated growth of organs is sufficient to simulate the development of maize plant structure without taking into account any regulation by assimilates. In this model, whole-plant architecture is shaped through initial conditions that feed a cascade of coordination events.
Figures


Similar articles
-
Early competition shapes maize whole-plant development in mixed stands.J Exp Bot. 2014 Feb;65(2):641-53. doi: 10.1093/jxb/ert408. Epub 2013 Dec 4. J Exp Bot. 2014. PMID: 24307719 Free PMC article.
-
Contrasting phenotypes emerging from stable rules: A model based on self-regulated control loops captures the dynamics of shoot extension in contrasting maize phenotypes.Ann Bot. 2020 Sep 14;126(4):615-633. doi: 10.1093/aob/mcz168. Ann Bot. 2020. PMID: 31630162 Free PMC article.
-
Onset of sheath extension and duration of lamina extension are major determinants of the response of maize lamina length to plant density.Ann Bot. 2006 Nov;98(5):1005-16. doi: 10.1093/aob/mcl177. Epub 2006 Aug 22. Ann Bot. 2006. PMID: 16926228 Free PMC article.
-
The integration of leaf-derived signals sets the timing of vegetative phase change in maize, a process coordinated by epigenetic remodeling.Plant Sci. 2021 Nov;312:111035. doi: 10.1016/j.plantsci.2021.111035. Epub 2021 Aug 27. Plant Sci. 2021. PMID: 34620439 Review.
-
The role of auxin in shaping shoot architecture.J Exp Bot. 2013 Jun;64(9):2593-608. doi: 10.1093/jxb/ert141. J Exp Bot. 2013. PMID: 23709672 Review.
Cited by
-
Effects of nitrogen and vapour pressure deficit on phytomer growth and development in a C4 grass.AoB Plants. 2017 Jan 2;8:plw075. doi: 10.1093/aobpla/plw075. Print 2016. AoB Plants. 2017. PMID: 27810947 Free PMC article.
-
A successive time-to-event model of phyllochron dynamics for hypothesis testing: application to the analysis of genetic and environmental effects in maize.Plant Methods. 2023 Jun 7;19(1):54. doi: 10.1186/s13007-023-01029-7. Plant Methods. 2023. PMID: 37287031 Free PMC article.
-
A Conserved Potential Development Framework Applies to Shoots of Legume Species with Contrasting Morphogenetic Strategies.Front Plant Sci. 2017 Mar 27;8:405. doi: 10.3389/fpls.2017.00405. eCollection 2017. Front Plant Sci. 2017. PMID: 28396676 Free PMC article.
-
Interactions Between Environment and Genetic Diversity in Perennial Grass Phenology: A Review of Processes at Plant Scale and Modeling.Front Plant Sci. 2021 Nov 16;12:672156. doi: 10.3389/fpls.2021.672156. eCollection 2021. Front Plant Sci. 2021. PMID: 34868095 Free PMC article. Review.
-
A unique approach to demonstrating that apical bud temperature specifically determines leaf initiation rate in the dicot Cucumis sativus.Planta. 2016 Apr;243(4):1071-9. doi: 10.1007/s00425-015-2464-4. Epub 2016 Jan 14. Planta. 2016. PMID: 26769623 Free PMC article.
References
-
- Birch CJ, Hammer GL, Rickert KG. Improved methods for predicting individual leaf area and leaf senescence in maize (Zea mays) Australian Journal of Agricultural Research. 1998;49:249–262.
-
- Buck-Sorlin GH, Kniemeyer O, Kurth W. Barley morphology, genetics and hormonal regulation of internode elongation modelled by a relational growth grammar. New Phytologist. 2005;166:859–867. - PubMed
-
- Casey IA, Brereton AJ, Laidlaw AS, McGilloway DA. Effects of sheath tube length on leaf development in perennial ryegrass (Lolium perenne L.) Annals of Applied Biology. 1999;134:251–257.
-
- Chuck G, Hake S. Regulation of developmental transitions. Current Opinion in Plant Biology. 2005;8:67–70. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

