Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study)
- PMID: 24748630
- PMCID: PMC4145441
- DOI: 10.1136/annrheumdis-2013-204902
Clinical efficacy, radiographic and safety findings through 5 years of subcutaneous golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of a randomised, placebo-controlled trial (the GO-REVEAL study)
Abstract
Objectives: Assess golimumab's long-term efficacy/safety in psoriatic arthritis (PsA).
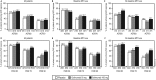
Methods: Adults with active PsA (≥3 swollen and tender joints, active psoriasis) were randomly assigned to subcutaneous placebo, golimumab 50 mg, or golimumab 100 mg every 4 weeks (q4wks) through wk20. All patients received golimumab 50 mg or 100 mg q4wks from wk24 forward. Methotrexate was allowed and taken by approximately half the patients. Findings through 5 years are reported herein. Efficacy assessments included ≥20% improvement in American College of Rheumatology (ACR20) response, C-reactive-protein-based, 28-joint-count Disease Activity Score (DAS28-CRP) response, ≥75% improvement in Psoriasis Area and Severity Index (PASI75) scores, and PsA-modified Sharp/van der Heijde scores (SHSs).
Results: 126/405 (31%) randomised patients discontinued treatment through wk252. Golimumab was effective in maintaining clinical improvement through year-5 (ACR20: 62.8-69.9%, DAS28-CRP: 75.2-84.9% for randomised patients; PASI75: 60.8-72.2% among randomised patients with ≥3% body surface area involvement) and inhibiting radiographic progression (mean changes in PsA-modified SHS: 0.1-0.3) among patients with radiographic data. While concomitant methotrexate did not affect ACR20/PASI75, it appeared to reduce radiographic progression. No new safety signals were identified. Antibodies-to-golimumab occurred in 1.8%/10.0% of patients with/without methotrexate).
Conclusions: Long-term golimumab safety/efficacy in PsA was demonstrated through 5 years.
Trial registration number: NCT00265096.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
Similar articles
-
Clinical efficacy, radiographic and safety findings through 2 years of golimumab treatment in patients with active psoriatic arthritis: results from a long-term extension of the randomised, placebo-controlled GO-REVEAL study.Ann Rheum Dis. 2013 Nov;72(11):1777-85. doi: 10.1136/annrheumdis-2012-202035. Epub 2012 Nov 17. Ann Rheum Dis. 2013. PMID: 23161902 Free PMC article. Clinical Trial.
-
Golimumab in psoriatic arthritis: one-year clinical efficacy, radiographic, and safety results from a phase III, randomized, placebo-controlled trial.Arthritis Rheum. 2012 Aug;64(8):2504-17. doi: 10.1002/art.34436. Arthritis Rheum. 2012. PMID: 22378566 Clinical Trial.
-
Radiographic benefit and maintenance of clinical benefit with intravenous golimumab therapy in patients with active rheumatoid arthritis despite methotrexate therapy: results up to 1 year of the phase 3, randomised, multicentre, double blind, placebo controlled GO-FURTHER trial.Ann Rheum Dis. 2014 Dec;73(12):2152-9. doi: 10.1136/annrheumdis-2013-203742. Epub 2013 Sep 3. Ann Rheum Dis. 2014. PMID: 24001888 Free PMC article. Clinical Trial.
-
Golimumab: in the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.BioDrugs. 2009;23(2):125-35. doi: 10.2165/00063030-200923020-00005. BioDrugs. 2009. PMID: 19489653 Review.
-
Golimumab: Review of the efficacy and tolerability of a recently approved tumor necrosis factor-α inhibitor.Clin Ther. 2010 Sep;32(10):1681-703. doi: 10.1016/j.clinthera.2010.09.003. Clin Ther. 2010. PMID: 21194591 Review.
Cited by
-
The role of golimumab in inflammatory arthritis. A review of the evidence.Ther Adv Musculoskelet Dis. 2018 Sep 6;10(9):181-194. doi: 10.1177/1759720X18793317. eCollection 2018 Sep. Ther Adv Musculoskelet Dis. 2018. PMID: 30210584 Free PMC article. Review.
-
Real-world treatment persistence of golimumab in the management of immune-mediated rheumatic diseases in Europe: a systematic literature review.BMJ Open. 2019 May 28;9(5):e027456. doi: 10.1136/bmjopen-2018-027456. BMJ Open. 2019. PMID: 31142529 Free PMC article.
-
Advances in the treatment of polyarticular juvenile idiopathic arthritis.Curr Opin Rheumatol. 2015 Sep;27(5):505-10. doi: 10.1097/BOR.0000000000000206. Curr Opin Rheumatol. 2015. PMID: 26147756 Free PMC article. Review.
-
Tailored treatment options for patients with psoriatic arthritis and psoriasis: review of established and new biologic and small molecule therapies.Rheumatol Int. 2016 May;36(5):603-12. doi: 10.1007/s00296-016-3436-0. Epub 2016 Feb 18. Rheumatol Int. 2016. PMID: 26892034 Free PMC article. Review.
-
Golimumab (anti-TNF monoclonal antibody): where we stand today.Hum Vaccin Immunother. 2021 Jun 3;17(6):1586-1598. doi: 10.1080/21645515.2020.1836919. Epub 2020 Dec 28. Hum Vaccin Immunother. 2021. PMID: 33369527 Free PMC article. Review.
References
-
- Antoni CE, Kavanaugh A, Kirkham B, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT). Arthritis Rheum 2005;52:1227–36 - PubMed
-
- Antoni CE, Kavanaugh A, van der Heijde D, et al. Two-year efficacy and safety of infliximab treatment in patients with active psoriatic arthritis: findings of the Infliximab Multinational Psoriatic Arthritis Controlled Trial (IMPACT). J Rheumatol 2008;35:869–76 - PubMed
-
- Mease PJ, Gladman DD, Ritchlin CT, et al. ; Adalimumab Effectiveness in Psoriatic Arthritis Trial Study Group. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89 - PubMed
-
- Mease PJ, Goffe BS, Metz J, et al. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomized trial. Lancet 2000;356:385–90 - PubMed
-
- Mease PJ, Kivitz AJ, Burch FX, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264–72 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

