Emergent complexity of the cytoskeleton: from single filaments to tissue
- PMID: 24748680
- PMCID: PMC3985726
- DOI: 10.1080/00018732.2013.771509
Emergent complexity of the cytoskeleton: from single filaments to tissue
Abstract
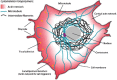
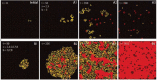
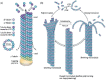
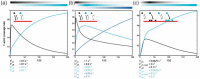
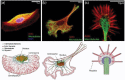
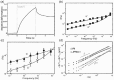
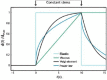
Despite their overwhelming complexity, living cells display a high degree of internal mechanical and functional organization which can largely be attributed to the intracellular biopolymer scaffold, the cytoskeleton. Being a very complex system far from thermodynamic equilibrium, the cytoskeleton's ability to organize is at the same time challenging and fascinating. The extensive amounts of frequently interacting cellular building blocks and their inherent multifunctionality permits highly adaptive behavior and obstructs a purely reductionist approach. Nevertheless (and despite the field's relative novelty), the physics approach has already proved to be extremely successful in revealing very fundamental concepts of cytoskeleton organization and behavior. This review aims at introducing the physics of the cytoskeleton ranging from single biopolymer filaments to multicellular organisms. Throughout this wide range of phenomena, the focus is set on the intertwined nature of the different physical scales (levels of complexity) that give rise to numerous emergent properties by means of self-organization or self-assembly.
Keywords: 87. Biological and medical physics; 87.16.-b Subcellular structure and processes; 87.16.Ln Cytoskeleton; 87.17.-d Cell processes; 87.17.Ee Growth and division; 87.17.Jj Cell locomotion, chemotaxis; 87.17.Rt Cell adhesion and cell mechanics; 87.18-h Biological complexity; 87.18.Ed Cell aggregation; 87.18.Fx Multicellular phenomena, biofilms; 87.18.Hf Spatiotemporal pattern formation in cellular populations; 87.19.xj Cancer; cell migration; cellular mechanics; emergent properties; multifunctionality; self-assembly; self-organization.
Figures











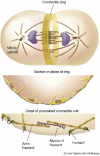
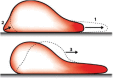
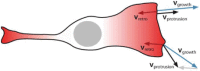


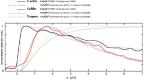

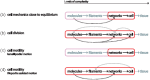
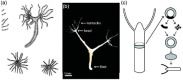
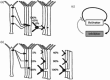
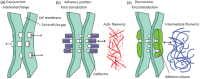


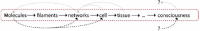
References
-
- Anderson P.W. Science. 1972;177:393–396. - PubMed
-
- Schrödinger E. What is Life? The Physical Aspect of the Living Cell. Cambridge: Cambridge University Press; 1944.
-
- Camazine S., Deneubourg J.-L., Franks N.R., Sneyd J., Theraula G., Bonabeau E. Self-Organization in Biological Systems. Princeton, NJ: Princeton University Press; 2003.
-
- Schaller V., Weber C., Semmrich C., Frey E., Bausch A.R. Nature. 2010;467:73–77. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
