Triazole derivatives with improved in vitro antifungal activity over azole drugs
- PMID: 24748772
- PMCID: PMC3986111
- DOI: 10.2147/DDDT.S58680
Triazole derivatives with improved in vitro antifungal activity over azole drugs
Abstract
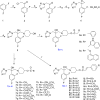
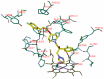
A series of triazole antifungal agents with piperidine side chains was designed and synthesized. The results of antifungal tests against eight human pathogenic fungi in vitro showed that all the compounds exhibited moderate-to-excellent activities. Molecular docking between 8d and the active site of Candida albicans CYP51 was provided based on the computational docking results. The triazole interacts with the iron of the heme group. The difluorophenyl group is located in the S3 subsite and its fluorine atom (2-F) can form H-bonds with Gly307. The side chain is oriented into the S4 subsite and formed hydrophobic and van der Waals interactions with the amino residues. Moreover, the phenyl group in the side chain interacts with the phenol group of Phe380 through the formation of π-π face-to-edge interactions.
Keywords: CYP51; azole agents; molecular docking; synthesis.
Figures
Similar articles
-
Synthesis and biological evaluation of triazole derivatives as potential antifungal agent.Chem Biol Drug Des. 2012 Sep;80(3):382-7. doi: 10.1111/j.1747-0285.2012.01398.x. Epub 2012 Jun 27. Chem Biol Drug Des. 2012. PMID: 22531027
-
Synthesis, In Vitro Biological Evaluation, and Molecular Docking of New Triazoles as Potent Antifungal Agents.Arch Pharm (Weinheim). 2016 Jan;349(1):42-9. doi: 10.1002/ardp.201500313. Epub 2015 Dec 7. Arch Pharm (Weinheim). 2016. PMID: 26641629
-
Novel conformationally restricted triazole derivatives with potent antifungal activity.Eur J Med Chem. 2010 Dec;45(12):6020-6. doi: 10.1016/j.ejmech.2010.09.070. Epub 2010 Oct 7. Eur J Med Chem. 2010. PMID: 20950895
-
Recent developments in azole compounds as antibacterial and antifungal agents.Curr Top Med Chem. 2013 Aug;13(16):1963-2010. doi: 10.2174/15680266113139990125. Curr Top Med Chem. 2013. PMID: 23895097 Review.
-
Azole Derivatives: Recent Advances as Potent Antibacterial and Antifungal Agents.Curr Med Chem. 2023;30(2):220-249. doi: 10.2174/0929867329666220407094430. Curr Med Chem. 2023. PMID: 35392780 Review.
Cited by
-
Design, synthesis, and antifungal activities of novel triazole derivatives containing the benzyl group.Drug Des Devel Ther. 2015 Mar 11;9:1459-67. doi: 10.2147/DDDT.S74989. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25792806 Free PMC article.
-
Triazolo Based-Thiadiazole Derivatives. Synthesis, Biological Evaluation and Molecular Docking Studies.Antibiotics (Basel). 2021 Jul 2;10(7):804. doi: 10.3390/antibiotics10070804. Antibiotics (Basel). 2021. PMID: 34356726 Free PMC article.
-
An insight on medicinal attributes of 1,2,4-triazoles.Eur J Med Chem. 2020 Nov 1;205:112652. doi: 10.1016/j.ejmech.2020.112652. Epub 2020 Jul 27. Eur J Med Chem. 2020. PMID: 32771798 Free PMC article. Review.
-
Chromenol Derivatives as Novel Antifungal Agents: Synthesis, In Silico and In Vitro Evaluation.Molecules. 2021 Jul 16;26(14):4304. doi: 10.3390/molecules26144304. Molecules. 2021. PMID: 34299579 Free PMC article.
-
Triazole-Modified Peptidomimetics: An Opportunity for Drug Discovery and Development.Front Chem. 2021 May 20;9:674705. doi: 10.3389/fchem.2021.674705. eCollection 2021. Front Chem. 2021. PMID: 34095086 Free PMC article. Review.
References
-
- Singh N. Trends in the epidemiology of opportunistic fungal infections: predisposing factors and the impact of antimicrobial use practices. Clin Infect Dis. 2001;33(10):1692–1696. - PubMed
-
- Ablordeppey SY, Fan P, Ablordeppey JH, Mardenborough L. Systemic antifungal agents against AIDS-related opportunistic infections: current status and emerging drugs in development. Curr Med Chem. 1999;6(12):1151–1195. - PubMed
-
- Wildfeuer A, Seidl HP, Paule I, Haberreiter A. In vitro evaluation of voriconazole against clinical isolates of yeasts, moulds and dermatophytes in comparison with itraconazole, ketoconazole, amphotericin B and griseofulvin. Mycoses. 1998;41(7–8):309–319. - PubMed
-
- Georgopapadakou NH. Antifungals: mechanism of action and resistance, established and novel drugs. Curr Opin Microbiol. 1998;1(5):547–557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

