Characterization of a fusion protein of RGD4C and the β-lactamase variant for antibody-directed enzyme prodrug therapy
- PMID: 24748803
- PMCID: PMC3986274
- DOI: 10.2147/OTT.S59346
Characterization of a fusion protein of RGD4C and the β-lactamase variant for antibody-directed enzyme prodrug therapy
Abstract
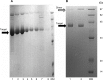
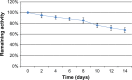
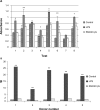
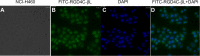
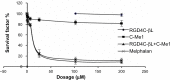
Antibody-directed enzyme prodrug therapy (ADEPT) delivers chemotherapeutic agents in high concentration to tumor tissue, while minimizing systemic drug exposure. ADEPT has been reported to be an attractive approach for improving the efficacy of cancer therapy. A previously reported β-lactamase was found to contain four cluster of differentiation (CD)4(+) T cell epitopes; however, single amino acid changes in the enzyme resulted in significantly reduced proliferative responses, while retaining stability and activity of the enzyme. The β-lactamase variant with reduced immunogenicity is an attractive alternative for constructing the ADEPT fusion protein. In this study, we fused the peptide, RGD4C, known to target integrin αvβ3, to the β-lactamase variant for use in ADEPT. Biological function studies revealed that RGD4C-β-lactamase had a high hydrolytic effect on nitrocefin and cephalosporin-melphalan, and high plasma stability was observed. In addition, fusion of the RGD4C moiety to β-lactamase had little effect on immunogenicity compared with β-lactamase in the proliferation of peripheral blood mononuclear cells. The ability of this fusion protein to both target the central region of αvβ3 and induce toxicity in the non-small-cell lung cancer cell NCI-H460 makes it a promising therapeutic approach in the treatment of cancer.
Keywords: ADEPT; immunogenicity; integrin αvβ3.
Figures
Similar articles
-
A Fusion Protein of RGD4C and β-Lactamase Has a Favorable Targeting Effect in Its Use in Antibody Directed Enzyme Prodrug Therapy.Int J Mol Sci. 2015 Apr 28;16(5):9625-34. doi: 10.3390/ijms16059625. Int J Mol Sci. 2015. PMID: 25927583 Free PMC article.
-
A beta-lactamase with reduced immunogenicity for the targeted delivery of chemotherapeutics using antibody-directed enzyme prodrug therapy.Mol Cancer Ther. 2005 Nov;4(11):1791-800. doi: 10.1158/1535-7163.MCT-05-0189. Mol Cancer Ther. 2005. PMID: 16276001
-
Construction, expression and functional characterization of the β-lactamase with αv integrin ligands.Protein Pept Lett. 2010 Dec;17(12):1562-5. doi: 10.2174/0929866511009011562. Protein Pept Lett. 2010. PMID: 20858201
-
Translating antibody directed enzyme prodrug therapy (ADEPT) and prospects for combination.Expert Opin Biol Ther. 2017 Jan;17(1):1-13. doi: 10.1080/14712598.2017.1247802. Epub 2016 Oct 24. Expert Opin Biol Ther. 2017. PMID: 27737561 Review.
-
Antibody-enzyme conjugates for cancer therapy.J Natl Cancer Inst. 1996 Feb 21;88(3-4):153-65. doi: 10.1093/jnci/88.3-4.153. J Natl Cancer Inst. 1996. PMID: 8632489 Review.
Cited by
-
Peptide-Based Drug Delivery Systems.Medicina (Kaunas). 2021 Nov 5;57(11):1209. doi: 10.3390/medicina57111209. Medicina (Kaunas). 2021. PMID: 34833427 Free PMC article. Review.
-
Targeting assay of a fusion protein applied in enzyme prodrug therapy.Oncol Lett. 2017 Apr;13(4):2698-2702. doi: 10.3892/ol.2017.5768. Epub 2017 Feb 22. Oncol Lett. 2017. PMID: 28454453 Free PMC article.
-
A Fusion Protein of RGD4C and β-Lactamase Has a Favorable Targeting Effect in Its Use in Antibody Directed Enzyme Prodrug Therapy.Int J Mol Sci. 2015 Apr 28;16(5):9625-34. doi: 10.3390/ijms16059625. Int J Mol Sci. 2015. PMID: 25927583 Free PMC article.
References
-
- Wang H, Chen K, Cai W, et al. Integrin-targeted imaging and therapy with RGD4C-TNF fusion protein. Mol Cancer Ther. 2008;7(5):1044–1053. - PubMed
-
- Tucker GC. Integrins: molecular targets in cancer therapy. Curr Oncol Rep. 2006;8(2):96–103. - PubMed
-
- Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology (N Y) 1995;13(3):265–270. - PubMed
-
- Kerr DE, Li Z, Siemers NO, Senter PD, Vrudhula VM. Development and activities of a new melphalan prodrug designed for tumor-selective activation. Bioconjug Chem. 1998;9(2):255–259. - PubMed
-
- Goding JW. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3–4):215–226. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

