Switching patients from other inhaled corticosteroid devices to the Easyhaler(®): historical, matched-cohort study of real-life asthma patients
- PMID: 24748807
- PMCID: PMC3986277
- DOI: 10.2147/JAA.S59386
Switching patients from other inhaled corticosteroid devices to the Easyhaler(®): historical, matched-cohort study of real-life asthma patients
Abstract
Purpose: To investigate the clinical and cost effectiveness of switching real-life asthma patients from other types of inhalers to the Easyhaler(®) (EH) for the administration of inhaled corticosteroids (ICS).
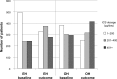
Patients and methods: Historical, matched-cohort study of 1,958 asthma patients (children and adults) treated in UK primary-care practices, using data obtained from the Optimum Patient Care Research Database and Clinical Practice Research Datalink. Other inhalers (OH) included pressurized metered-dose inhalers, breath-actuated inhalers, and dry-powder inhalers, delivering beclomethasone, budesonide, fluticasone, or ciclesonide. Patients remaining on OH unchanged (same drug, dosage, and device; n=979) were matched 1:1 with those switched to the EH (beclomethasone or budesonide) at the same or lower ICS dosage (n=979), based on age, sex, year of index patient review/switch, most recent ICS drug, dosage, and device, and the number of severe exacerbations and average daily short-acting β2 agonist (SABA) dosage in the preceding year. Clinical outcomes and health care costs were compared between groups for 12 months before and after the switch. Co-primary clinical outcomes were: 1) risk domain asthma control (RDAC) - no asthma-related hospitalization, acute oral steroid use, or lower respiratory tract infection (LRTI); 2) exacerbation rate (American Thoracic Society [ATS] definition) - where exacerbation is asthma-related hospitalization or acute oral steroid use; 3) exacerbation rate (clinical definition) - where exacerbation is ATS exacerbation or LRTI; and 4) overall asthma control (OAC) - RDAC plus average salbutamol-equivalent SABA dosage ≤200 μg/day. Non-inferiority (at least equivalence) of EH was tested against OH for the four co-primary outcomes in order (hierarchical approach) by comparing the difference in proportions of patients [EH-OH] achieving asthma control or having no exacerbations in the outcome year, using a limit of 10% difference.
Results: Non-inferiority was shown for the EH for all four co-primary outcomes. There were no significant differences between groups for RDAC or exacerbation rates, but EH patients were significantly more likely to achieve OAC (adjusted odds ratio [95% confidence interval]: 1.26 [1.05, 1.52]), as significantly more EH than OH patients had an average SABA dosage of ≤200 μg/day (52% versus 47%, respectively; P<0.001). Mean asthma-related health care costs increased from baseline to outcome years in both groups, but SABA costs increased significantly more in OH than EH patients (mean difference £5.5/patient/year) and consultation costs decreased significantly more in EH than OH patients (mean difference £13.5/patient/year).
Conclusion: Typical asthma patients may be switched from other ICS devices to the Easyhaler(®) with no reduction in clinical effectiveness or increase in cost.
Keywords: Easyhaler; ICS; asthma; cost; inhaler.
Figures


Similar articles
-
Real-life effectiveness of inhaler device switch from dry powder inhalers to pressurized metred-dose inhalers in patients with asthma treated with ICS/LABA.Respirology. 2019 Oct;24(10):972-979. doi: 10.1111/resp.13559. Epub 2019 Apr 30. Respirology. 2019. PMID: 31038269
-
Clinical and cost effectiveness of switching asthma patients from fluticasone-salmeterol to extra-fine particle beclometasone-formoterol: a retrospective matched observational study of real-world patients.Prim Care Respir J. 2013 Dec;22(4):439-48. doi: 10.4104/pcrj.2013.00088. Prim Care Respir J. 2013. PMID: 24186700 Free PMC article.
-
Effectiveness of initiating extrafine-particle versus fine-particle inhaled corticosteroids as asthma therapy in the Netherlands.BMC Pulm Med. 2016 May 17;16(1):80. doi: 10.1186/s12890-016-0234-0. BMC Pulm Med. 2016. PMID: 27184175 Free PMC article.
-
An innovative corticosteroid/long-acting β2-agonist breath-triggered inhaler: facilitating lung delivery of fluticasone propionate/formoterol fumarate for the treatment of asthma.Expert Opin Drug Deliv. 2019 Dec;16(12):1367-1380. doi: 10.1080/17425247.2019.1689957. Epub 2019 Nov 28. Expert Opin Drug Deliv. 2019. PMID: 31752560 Review.
-
Systematic review of clinical effectiveness of pressurised metered dose inhalers versus other hand held inhaler devices for delivering corticosteroids in asthma.BMJ. 2001 Oct 20;323(7318):896-900. doi: 10.1136/bmj.323.7318.896. BMJ. 2001. PMID: 11668133 Free PMC article.
Cited by
-
Switching to the Dry-Powder Inhaler Easyhaler®: A Narrative Review of the Evidence.Pulm Ther. 2021 Dec;7(2):409-427. doi: 10.1007/s41030-021-00174-5. Epub 2021 Sep 27. Pulm Ther. 2021. PMID: 34581994 Free PMC article. Review.
-
Dose uniformity of budesonide Easyhaler® under simulated real-life conditions and with low inspiration flow rates.Chron Respir Dis. 2018 Aug;15(3):265-271. doi: 10.1177/1479972317745733. Epub 2017 Dec 7. Chron Respir Dis. 2018. PMID: 29216744 Free PMC article.
-
Real-world effectiveness evaluation of budesonide/formoterol Spiromax for the management of asthma and chronic obstructive pulmonary disease in the UK.BMJ Open. 2018 Oct 27;8(10):e022051. doi: 10.1136/bmjopen-2018-022051. BMJ Open. 2018. PMID: 30368448 Free PMC article.
-
The REal Life EVidence AssessmeNt Tool (RELEVANT): development of a novel quality assurance asset to rate observational comparative effectiveness research studies.Clin Transl Allergy. 2019 Mar 27;9:21. doi: 10.1186/s13601-019-0256-9. eCollection 2019. Clin Transl Allergy. 2019. PMID: 30962876 Free PMC article.
-
Performance of database-derived severe exacerbations and asthma control measures in asthma: responsiveness and predictive utility in a UK primary care database with linked questionnaire data.Pragmat Obs Res. 2018 Aug 10;9:29-42. doi: 10.2147/POR.S151615. eCollection 2018. Pragmat Obs Res. 2018. PMID: 30127653 Free PMC article.
References
-
- Aylin P, Bottle A, Jen MH, et al. HSMR mortality indicators. London: Doctor Foster Research; 2010. [Accessed on March 15, 2013]. Available from http://www.nhs.uk/scorecard/Documents/HSMR%20methodology%2009%20November....
-
- Electronic Medicines Compendium (eMC) Surrey: Datapharm Communications Ltd; 2012. [Accessed December 8, 2013]. Available from: http://www.medicines.org.uk/emc/searchresults.aspx?term=Easyhaler&search....
-
- Jäger L, Laurikainen K, Leinonen M, Silvasti M. Beclomethasone dipropionate Easyhaler is as effective as budesonide Turbohaler in the control of asthma and is preferred by patients. German Study Group. Int J Clin Pract. 2000;54(6):368–372. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

