Doxylamine succinate-pyridoxine hydrochloride (Diclegis) for the management of nausea and vomiting in pregnancy: an overview
- PMID: 24748822
- PMCID: PMC3990370
- DOI: 10.2147/IJWH.S46653
Doxylamine succinate-pyridoxine hydrochloride (Diclegis) for the management of nausea and vomiting in pregnancy: an overview
Abstract
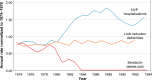
Nausea and vomiting in pregnancy (NVP) is common and often undertreated, in part due to fears of adverse effects of medications on the fetus during early pregnancy. In April 2013, the US Food and Drug Administration (FDA) approved doxylamine succinate 10 mg and pyridoxine hydrochloride (a vitamin B6 analog) 10 mg as a delayed-release combination pill called Diclegis for the treatment of NVP. Diclegis is currently the only medication that is FDA-approved for the indication of NVP. This review addresses the historical context, safety, efficacy, pharmacology, and practical role of doxylamine and pyridoxine for the management of NVP. The reintroduction of this doxylamine-pyridoxine combination pill into the American market fills a therapeutic gap in the management of NVP left by the removal of the same active drugs marketed over 30 years ago in the form of Bendectin. The substantial amount of safety data accumulated over the years makes it one of the few drugs that qualify for FDA Pregnancy Category A status. In the hierarchical approach to pharmacological treatment of NVP, the combination of doxylamine and pyridoxine should thus be first-tier.
Keywords: doxylamine; nausea; pregnancy; pyridoxine; vitamin B6; vomiting.
Figures
References
-
- Einarson TR, Piwko C, Koren G. Prevalence of nausea and vomiting of pregnancy in the USA: a meta analysis. J Popul Ther Clin Pharmacol. 2013;20(2):e163–e170. - PubMed
-
- Piwko C, Koren G, Babashov V, Vicente C, Einarson TR. Economic burden of nausea and vomiting of pregnancy in the USA. J Popul Ther Clin Pharmacol. 2013;20(2):e149–e160. - PubMed
-
- Lacroix R, Eason E, Melzack R. Nausea and vomiting during pregnancy: a prospective study of its frequency, intensity, and patterns of change. Am J Obstet Gynecol. 2000;182(4):931–937. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

