Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting
- PMID: 24748963
- PMCID: PMC3987918
- DOI: 10.1039/C3BM60274E
Hyaluronic acid hydrogel stiffness and oxygen tension affect cancer cell fate and endothelial sprouting
Abstract
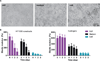
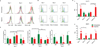
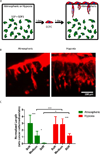
Three-dimensional (3D) tissue culture models may recapitulate aspects of the tumorigenic microenvironment in vivo, enabling the study of cancer progression in vitro. Both hypoxia and matrix stiffness are known to regulate tumor growth. Using a modular culture system employing an acrylated hyaluronic acid (AHA) hydrogel, three hydrogel matrices with distinctive degrees of viscoelasticity - soft (78±16 Pa), medium (309± 57 Pa), and stiff (596± 73 Pa) - were generated using the same concentration of adhesion ligands. Oxygen levels within the hydrogel in atmospheric (21 %), hypoxic (5 %), and severely hypoxic (1 %) conditions were assessed with a mathematical model. HT1080 fibrosarcoma cells, encapsulated within the AHA hydrogels in high densities, generated nonuniform oxygen distributions, while lower cell densities resulted in more uniform oxygen distributions in the atmospheric and hypoxic environments. When we examined how varying viscoelasticity in atmospheric and hypoxic environments affects cell cycles and the expression of BNIP3 and BNIP3L (autophagy and apoptosis genes), and GLUT-1 (a glucose transport gene), we observed that HT1080 cells in 3D hydrogel adapted better to hypoxic conditions than those in a Petri dish, with no obvious correlation to matrix viscoelasticity, by recovering rapidly from possible autophagy/apoptotic events and alternating metabolism mechanisms. Further, we examined how HT1080 cells cultured in varying viscoelasticity and oxygen tension conditions affected endothelial sprouting and invasion. We observed that increased matrix stiffness reduced endothelial sprouting and invasion in atmospheric conditions; however, we observed increased endothelial sprouting and invasion under hypoxia at all levels of matrix stiffness with the upregulation of vascular endothelial growth factor (VEGF) and angiopoeitin-1 (ANG-1). Overall, HT1080 cells encapsulated in the AHA hydrogels under hypoxic stress recovered better from apoptosis and demonstrated greater angiogenic induction. Thus, we propose that oxygen tension more profoundly influences cell fate and the angiogenic potential of 3D cultured HT1080 fibrosarcoma cells than does matrix stiffness.
Figures



Similar articles
-
Adaptation to oxygen deprivation in cultures of human pluripotent stem cells, endothelial progenitor cells, and umbilical vein endothelial cells.Am J Physiol Cell Physiol. 2010 Jun;298(6):C1527-37. doi: 10.1152/ajpcell.00484.2009. Epub 2010 Feb 24. Am J Physiol Cell Physiol. 2010. PMID: 20181925 Free PMC article.
-
Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEG-based hydrogels.Mol Pharm. 2014 Jul 7;11(7):2115-25. doi: 10.1021/mp5000828. Epub 2014 Apr 29. Mol Pharm. 2014. PMID: 24712441
-
Piceatannol-3-O-β-D-glucopyranoside (PG) exhibits in vitro anti-metastatic and anti-angiogenic activities in HT1080 malignant fibrosarcoma cells.Phytomedicine. 2019 Apr;57:95-104. doi: 10.1016/j.phymed.2018.12.017. Epub 2018 Dec 11. Phytomedicine. 2019. PMID: 30668328
-
[Research progress of matrix stiffness in regulating endothelial cell sprouting].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2023 Feb 15;37(2):202-207. doi: 10.7507/1002-1892.202210019. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2023. PMID: 36796817 Free PMC article. Review. Chinese.
-
Endothelial cell responses to hypoxic stress.Clin Exp Pharmacol Physiol. 1999 Jan;26(1):74-84. doi: 10.1046/j.1440-1681.1999.02992.x. Clin Exp Pharmacol Physiol. 1999. PMID: 10027074 Review.
Cited by
-
Biophysics Role and Biomimetic Culture Systems of ECM Stiffness in Cancer EMT.Glob Chall. 2022 Mar 20;6(6):2100094. doi: 10.1002/gch2.202100094. eCollection 2022 Jun. Glob Chall. 2022. PMID: 35712024 Free PMC article. Review.
-
The case for cancer-associated fibroblasts: essential elements in cancer drug discovery?Future Drug Discov. 2022 Jan;4(1):FDD71. doi: 10.4155/fdd-2021-0004. Epub 2021 Mar 30. Future Drug Discov. 2022. PMID: 35600290 Free PMC article. Review.
-
Soft Substrates Containing Hyaluronan Mimic the Effects of Increased Stiffness on Morphology, Motility, and Proliferation of Glioma Cells.Biomacromolecules. 2017 Oct 9;18(10):3040-3051. doi: 10.1021/acs.biomac.7b00324. Epub 2017 Sep 14. Biomacromolecules. 2017. PMID: 28858529 Free PMC article.
-
PEG-chitosan hydrogel with tunable stiffness for study of drug response of breast cancer cells.Polymers (Basel). 2016;8(4):112. doi: 10.3390/polym8040112. Epub 2016 Mar 26. Polymers (Basel). 2016. PMID: 27595012 Free PMC article.
-
For whom the cells pull: Hydrogel and micropost devices for measuring traction forces.Methods. 2016 Feb 1;94:51-64. doi: 10.1016/j.ymeth.2015.08.005. Epub 2015 Aug 8. Methods. 2016. PMID: 26265073 Free PMC article. Review.
References
-
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

