Developing a Model of the Benefits and Burdens of Research Participation in Cancer Clinical Trials
- PMID: 24748992
- PMCID: PMC3989990
- DOI: 10.1080/21507716.2011.653472
Developing a Model of the Benefits and Burdens of Research Participation in Cancer Clinical Trials
Abstract
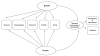
Background: Recruiting and retaining human participants in cancer clinical trials is challenging for many investigators. Although we expect participants to identify and weigh the benefits and burdens of research participation for themselves, it is not clear what burdens adult cancer participants perceive in relation to benefits. We identify key attributes and develop an initial conceptual framework of benefit and burden based on interviews with individuals enrolled in cancer clinical research.
Methods: Semistructured interviews were conducted with a purposive sample of 32 patients enrolled in cancer clinical trials at a large northeastern cancer center. Krueger's guidelines for qualitative methodology were followed.
Results: Respondents reported a range of benefits and burdens associated with research participation. Benefits such as access to needed medications that subjects otherwise might not be able to afford, early detection and monitoring of the disease, potential for remission or cure, and the ability to take control of their lives through actively participating in the trial were identified. Burdens included the potentiality of side effects, worry and fear of the unknown, loss of job support, and financial concerns.
Conclusions: Both benefit and burden influence research participation, including recruitment and retention in clinical trials. Dimensions of benefit and burden include physical, psychological, economic, familial, and social. Understanding the benefit-burden balance involved in the voluntary consent of human subjects is a fundamental tenet of research and important to ensure that subjects have made an informed decision regarding their decision to participate in clinical research.
Keywords: benefits; burdens; cancer; clinical trials; qualitative methods.
Figures
Similar articles
-
Perceived Benefits and Burdens of Participation for Caregivers of Cancer Patients in Hospice Clinical Trials: A Pilot Study.J Pain Symptom Manage. 2021 Jun;61(6):1147-1154. doi: 10.1016/j.jpainsymman.2020.10.024. Epub 2020 Nov 6. J Pain Symptom Manage. 2021. PMID: 33166583 Free PMC article. Clinical Trial.
-
Association of Perceived Benefit or Burden of Research Participation With Participants' Withdrawal From Cancer Clinical Trials.JAMA Netw Open. 2022 Nov 1;5(11):e2244412. doi: 10.1001/jamanetworkopen.2022.44412. JAMA Netw Open. 2022. PMID: 36449287 Free PMC article.
-
Participants' perceptions support the coexistence of benefits and burdens of cancer clinical trial participation.J Psychosoc Oncol. 2025;43(1):88-104. doi: 10.1080/07347332.2024.2366996. Epub 2024 Jun 22. J Psychosoc Oncol. 2025. PMID: 38907623
-
Strategies for research participant engagement: A synthetic review and conceptual framework.Clin Trials. 2021 Aug;18(4):457-465. doi: 10.1177/17407745211011068. Epub 2021 May 20. Clin Trials. 2021. PMID: 34011179 Review.
-
Involving South Asian patients in clinical trials.Health Technol Assess. 2004 Oct;8(42):iii, 1-109. doi: 10.3310/hta8420. Health Technol Assess. 2004. PMID: 15488164 Review.
Cited by
-
Cancer Clinical Trial Patient-Participants' Perceptions about Provider Communication and Dropout Intentions.AJOB Empir Bioeth. 2019 Jul-Sep;10(3):190-200. doi: 10.1080/23294515.2019.1618417. Epub 2019 Jun 10. AJOB Empir Bioeth. 2019. PMID: 31180295 Free PMC article.
-
Addressing Statistical Power and Increasing Diversity in Hospice Research: Electronic Medical Record Participant Identification Compared to Nurse Referral Approaches to Recruitment.J Pain Symptom Manage. 2024 Dec;68(6):594-602. doi: 10.1016/j.jpainsymman.2024.08.005. Epub 2024 Aug 26. J Pain Symptom Manage. 2024. PMID: 39197694
-
Informed Consent among Clinical Trial Participants with Different Cancer Diagnoses.AJOB Empir Bioeth. 2024 Jul-Sep;15(3):165-177. doi: 10.1080/23294515.2023.2262992. Epub 2023 Nov 3. AJOB Empir Bioeth. 2024. PMID: 37921867 Free PMC article.
-
Systematic review: bioethical implications for COVID-19 research in low prevalence countries, a distinctly different set of problems.BMC Med Ethics. 2021 Mar 3;22(1):22. doi: 10.1186/s12910-021-00589-4. BMC Med Ethics. 2021. PMID: 33658019 Free PMC article.
-
Cancer clinical trial participants' assessment of risk and benefit.AJOB Empir Bioeth. 2016;7(1):8-16. doi: 10.1080/23294515.2015.1034381. Epub 2015 May 1. AJOB Empir Bioeth. 2016. PMID: 26709381 Free PMC article.
References
-
- Advani AS, Atkeson B, Brown CL, et al. Barriers to the participation of African-American patients with cancer in clinical trials. Cancer. 2003;97:1499–1506. doi: 10.1002/cncr.11213. - PubMed
-
- Appelbaum PS, Roth LH, Lidz C. The therapeutic misconception: Informed consent in psychiatric research. International Journal of Law and Psychiatry. 1982;5:319–29. - PubMed
-
- Appelbaum PS, Roth LH, Lidz C, Benson P, Winslade W. False hopes and bestdata: Consent to research and the therapeutic misconception. Hastings Center Report. 1987;17:20–4. - PubMed
-
- Bradburn N. Respondent burden. Presented at Health survey research methods: Second biennial conference (Williamsburg, VA); Washington, DC. 1977.
Grants and funding
LinkOut - more resources
Full Text Sources