Developing a Model of the Benefits and Burdens of Research Participation in Cancer Clinical Trials
- PMID: 24748992
- PMCID: PMC3989990
- DOI: 10.1080/21507716.2011.653472
Developing a Model of the Benefits and Burdens of Research Participation in Cancer Clinical Trials
Abstract
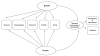
Background: Recruiting and retaining human participants in cancer clinical trials is challenging for many investigators. Although we expect participants to identify and weigh the benefits and burdens of research participation for themselves, it is not clear what burdens adult cancer participants perceive in relation to benefits. We identify key attributes and develop an initial conceptual framework of benefit and burden based on interviews with individuals enrolled in cancer clinical research.
Methods: Semistructured interviews were conducted with a purposive sample of 32 patients enrolled in cancer clinical trials at a large northeastern cancer center. Krueger's guidelines for qualitative methodology were followed.
Results: Respondents reported a range of benefits and burdens associated with research participation. Benefits such as access to needed medications that subjects otherwise might not be able to afford, early detection and monitoring of the disease, potential for remission or cure, and the ability to take control of their lives through actively participating in the trial were identified. Burdens included the potentiality of side effects, worry and fear of the unknown, loss of job support, and financial concerns.
Conclusions: Both benefit and burden influence research participation, including recruitment and retention in clinical trials. Dimensions of benefit and burden include physical, psychological, economic, familial, and social. Understanding the benefit-burden balance involved in the voluntary consent of human subjects is a fundamental tenet of research and important to ensure that subjects have made an informed decision regarding their decision to participate in clinical research.
Keywords: benefits; burdens; cancer; clinical trials; qualitative methods.
Figures
References
-
- Advani AS, Atkeson B, Brown CL, et al. Barriers to the participation of African-American patients with cancer in clinical trials. Cancer. 2003;97:1499–1506. doi: 10.1002/cncr.11213. - PubMed
-
- Appelbaum PS, Roth LH, Lidz C. The therapeutic misconception: Informed consent in psychiatric research. International Journal of Law and Psychiatry. 1982;5:319–29. - PubMed
-
- Appelbaum PS, Roth LH, Lidz C, Benson P, Winslade W. False hopes and bestdata: Consent to research and the therapeutic misconception. Hastings Center Report. 1987;17:20–4. - PubMed
-
- Bradburn N. Respondent burden. Presented at Health survey research methods: Second biennial conference (Williamsburg, VA); Washington, DC. 1977.
Grants and funding
LinkOut - more resources
Full Text Sources