Clinical course of infliximab treatment in korean pediatric ulcerative colitis patients: a single center experience
- PMID: 24749085
- PMCID: PMC3990780
- DOI: 10.5223/pghn.2014.17.1.31
Clinical course of infliximab treatment in korean pediatric ulcerative colitis patients: a single center experience
Abstract
Purpose: Infliximab (IFX) is considered safe and effective for the treatment of ulcerative colitis (UC) in both adults and children. The aim of this study was to evaluate the short- and long-term clinical course of IFX in Korean children with UC.
Methods: Pediatric patients with UC who had received IFX infusions between November 2007 and May 2013 at Samsung Medical Center were retrospectively investigated. The clinical efficacy of IFX treatment was evaluated at 8 weeks (short term) and 54 weeks (long term) after the initiation of IFX treatment using the Pediatric Ulcerative Colitis Activity Index (PUCAI). The degree of response to IFX treatment was defined as complete response (PUCAI score=0), partial response (decrement of PUCAI score≥20 points), and non-response (decrement of PUCAI score <20 points). Adverse events associated with IFX treatment were also investigated.
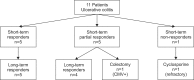
Results: Eleven pediatric patients with moderate to severe UC had received IFX. The remission rate after IFX treatment was 46% (5/11) and 82% (9/11) at 8 weeks and 54 weeks after IFX treatment, respectively. All patients who were steroid-dependent before treatment with IFX achieved remission at 54 weeks and were able to stop treatment with corticosteroids, while all steroid-refractory patients failed to achieve remission at 54 weeks after treatment with IFX.
Conclusion: Response to IFX treatment after 8 weeks may predict a favorable long-term response to IFX treatment in Korean pediatric UC patients.
Keywords: Child; Inflammatory bowel diseases; Infliximab; Korea; Ulcerative colitis.
Figures
References
-
- Baldassano RN, Piccoli DA. Inflammatory bowel disease in pediatric and adolescent patients. Gastroenterol Clin North Am. 1999;28:445–458. - PubMed
-
- Kader HA, Mascarenhas MR, Piccoli DA, Stouffer NO, Baldassano RN. Experiences with 6-mercaptopurine and azathioprine therapy in pediatric patients with severe ulcerative colitis. J Pediatr Gastroenterol Nutr. 1999;28:54–58. - PubMed
-
- Nielsen OH, Vainer B, Rask-Madsen J. Review article: the treatment of inflammatory bowel disease with 6-mercaptopurine or azathioprine. Aliment Pharmacol Ther. 2001;15:1699–1708. - PubMed
-
- Paoluzi OA, Pica R, Marcheggiano A, Crispino P, Iacopini F, Iannoni C, et al. Azathioprine or methotrexate in the treatment of patients with steroid-dependent or steroid-resistant ulcerative colitis: results of an open-label study on efficacy and tolerability in inducing and maintaining remission. Aliment Pharmacol Ther. 2002;16:1751–1759. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources