Alveolar macrophages contribute to the pathogenesis of human metapneumovirus infection while protecting against respiratory syncytial virus infection
- PMID: 24749674
- PMCID: PMC4189482
- DOI: 10.1165/rcmb.2013-0414OC
Alveolar macrophages contribute to the pathogenesis of human metapneumovirus infection while protecting against respiratory syncytial virus infection
Abstract
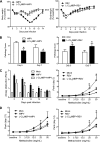
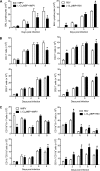
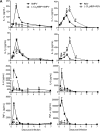
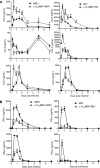
Human metapneumovirus (hMPV) and respiratory syncytial virus (RSV) are leading causes of upper and lower respiratory tract infections in young children and among elderly and immunocompromised patients. The pathogenesis of hMPV-induced lung disease is poorly understood. The lung macrophage population consists of alveolar macrophages (AMs) residing at the luminal surface of alveoli and interstitial macrophages present within the parenchymal lung interstitium. The involvement of AMs in innate immune responses to virus infections remains elusive. In this study, BALB/c mice depleted of AMs by intranasal instillation of dichloromethylene bisphosphonate (L-CL2MBP) liposomes were examined for disease, lung inflammation, and viral replication after infection with hMPV or RSV. hMPV-infected mice lacking AMs exhibited improved disease in terms of body weight loss, lung inflammation, airway obstruction, and hyperresponsiveness compared with AM-competent mice. AM depletion was associated with significantly reduced hMPV titers in the lungs, suggesting that hMPV required AMs for early entry and replication in the lung. In contrast, AM depletion in the context of RSV infection was characterized by an increase in viral replication, worsened disease, and inflammation, with increased airway neutrophils and inflammatory dendritic cells. Overall, lack of AMs resulted in a broad-spectrum disruption in type I IFN and certain inflammatory cytokine production, including TNF and IL-6, while causing a virus-specific alteration in the profile of several immunomodulatory cytokines, chemokines, and growth factors. Our study demonstrates that AMs have distinct roles in the context of human infections caused by members of the Paramyxoviridae family.
Keywords: alveolar macrophages; human metapneumovirus; pathogenesis; respiratory syncytial virus; viral infection.
Figures

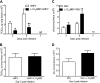
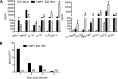
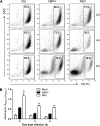
Similar articles
-
Human metapneumovirus induces more severe disease and stronger innate immune response in BALB/c mice as compared with respiratory syncytial virus.Respir Res. 2007 Jan 29;8(1):6. doi: 10.1186/1465-9921-8-6. Respir Res. 2007. PMID: 17257445 Free PMC article.
-
Contribution of NKT cells to the immune response and pathogenesis triggered by respiratory viruses.Virulence. 2020 Dec;11(1):580-593. doi: 10.1080/21505594.2020.1770492. Virulence. 2020. PMID: 32463330 Free PMC article.
-
Protective Role of Nuclear Factor Erythroid 2-Related Factor 2 Against Respiratory Syncytial Virus and Human Metapneumovirus Infections.Front Immunol. 2018 Apr 23;9:854. doi: 10.3389/fimmu.2018.00854. eCollection 2018. Front Immunol. 2018. PMID: 29740449 Free PMC article.
-
Aberrant T cell immunity triggered by human Respiratory Syncytial Virus and human Metapneumovirus infection.Virulence. 2017 Aug 18;8(6):685-704. doi: 10.1080/21505594.2016.1265725. Epub 2016 Dec 2. Virulence. 2017. PMID: 27911218 Free PMC article. Review.
-
The distinguishing features of human metapneumovirus and respiratory syncytial virus.Rev Med Virol. 2010 Jul;20(4):245-60. doi: 10.1002/rmv.651. Rev Med Virol. 2010. PMID: 20586081 Review.
Cited by
-
Differential mucin expression by respiratory syncytial virus and human metapneumovirus infection in human epithelial cells.Mediators Inflamm. 2015;2015:347292. doi: 10.1155/2015/347292. Epub 2015 Apr 22. Mediators Inflamm. 2015. PMID: 25977598 Free PMC article.
-
Host Responses to Respiratory Syncytial Virus Infection.Viruses. 2023 Sep 26;15(10):1999. doi: 10.3390/v15101999. Viruses. 2023. PMID: 37896776 Free PMC article. Review.
-
Blood myeloid cells differentiate to lung resident cells and respond to pathogen stimuli in a 3D human tissue-engineered lung model.Front Bioeng Biotechnol. 2023 Jul 7;11:1212230. doi: 10.3389/fbioe.2023.1212230. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37485324 Free PMC article.
-
TNF-mediated alveolar macrophage necroptosis drives disease pathogenesis during respiratory syncytial virus infection.Eur Respir J. 2021 Jun 17;57(6):2003764. doi: 10.1183/13993003.03764-2020. Print 2021 Jun. Eur Respir J. 2021. PMID: 33303545 Free PMC article.
-
M1-like, but not M0- or M2-like, macrophages, reduce RSV infection of primary bronchial epithelial cells in a media-dependent fashion.PLoS One. 2022 Oct 13;17(10):e0276013. doi: 10.1371/journal.pone.0276013. eCollection 2022. PLoS One. 2022. PMID: 36228018 Free PMC article.
References
-
- Kahn JS. Human metapneumovirus: a newly emerging respiratory pathogen. Curr Opin Infect Dis. 2003;16:255–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

