Repeat treatment of acute hereditary angioedema attacks with open-label icatibant in the FAST-1 trial
- PMID: 24749847
- PMCID: PMC4226605
- DOI: 10.1111/cei.12358
Repeat treatment of acute hereditary angioedema attacks with open-label icatibant in the FAST-1 trial
Abstract
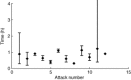
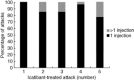
Hereditary angioedema (HAE) is characterized by potentially life-threatening recurrent episodes of oedema. The open-label extension (OLE) phase of the For Angioedema Subcutaneous Treatment (FAST)-1 trial (NCT00097695) evaluated the efficacy and safety of repeated icatibant exposure in adults with multiple HAE attacks. Following completion of the randomized, controlled phase, patients could receive open-label icatibant (30 mg subcutaneously) for subsequent attacks. The primary end-point was time to onset of primary symptom relief, as assessed by visual analogue scale (VAS). Descriptive statistics were reported for cutaneous/abdominal attacks 1-10 treated in the OLE phase and individual laryngeal attacks. Post-hoc analyses were conducted in patients with ≥ 5 attacks across the controlled and OLE phases. Safety was evaluated throughout. During the OLE phase, 72 patients received icatibant for 340 attacks. For cutaneous/abdominal attacks 1-10, the median time to onset of primary symptom relief was 1·0-2·0 h. For laryngeal attacks 1-12, patient-assessed median time to initial symptom improvement was 0·3-1·2 h. Post-hoc analyses showed the time to onset of symptom relief based on composite VAS was consistent across repeated treatments with icatibant. One injection of icatibant was sufficient to treat 88·2% of attacks; rescue medication was required in 5·3% of attacks. No icatibant-related serious adverse events were reported. Icatibant provided consistent efficacy and was well tolerated for repeated treatment of HAE attacks.
Keywords: C1-inhibitor deficiency; FAST-1; OLE phase; bradykinin B2 receptor antagonist; hereditary angioedema; icatibant.
© 2014 British Society for Immunology.
Figures

References
-
- Zuraw BL. Hereditary angioedema. N Engl J Med. 2008;359:1027–1036. - PubMed
-
- Weis M. Clinical review of hereditary angioedema: diagnosis and management. Postgrad Med. 2009;121:113–120. - PubMed
-
- Bork K, Harct J, Schicketanz K-H, Ressel N. Clinical studies of sudden upper airway obstruction in patients with hereditary angioedema due to C1 esterase inhibitor deficiency. Arch Intern Med. 2003;163:1229–1235. - PubMed
-
- Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1-INH deficiency. J Allergy Clin Immunol. 2012;130:692–697. - PubMed
-
- Winnewisser J, Rossi M, Spath P, Bürgi H. Type I hereditary angio-oedema. Variability of clinical presentation and course within two large kindreds. J Intern Med. 1997;241:39–46. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

