3-Substituted-N-(4-hydroxynaphthalen-1-yl)arylsulfonamides as a novel class of selective Mcl-1 inhibitors: structure-based design, synthesis, SAR, and biological evaluation
- PMID: 24749893
- PMCID: PMC4033665
- DOI: 10.1021/jm500010b
3-Substituted-N-(4-hydroxynaphthalen-1-yl)arylsulfonamides as a novel class of selective Mcl-1 inhibitors: structure-based design, synthesis, SAR, and biological evaluation
Abstract
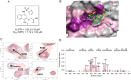
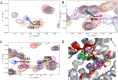
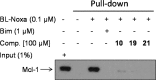
Mcl-1, an antiapoptotic member of the Bcl-2 family of proteins, is a validated and attractive target for cancer therapy. Overexpression of Mcl-1 in many cancers results in disease progression and resistance to current chemotherapeutics. Utilizing high-throughput screening, compound 1 was identified as a selective Mcl-1 inhibitor and its binding to the BH3 binding groove of Mcl-1 was confirmed by several different, but complementary, biochemical and biophysical assays. Guided by structure-based drug design and supported by NMR experiments, comprehensive SAR studies were undertaken and a potent and selective inhibitor, compound 21, was designed which binds to Mcl-1 with a Ki of 180 nM. Biological characterization of 21 showed that it disrupts the interaction of endogenous Mcl-1 and biotinylated Noxa-BH3 peptide, causes cell death through a Bak/Bax-dependent mechanism, and selectively sensitizes Eμ-myc lymphomas overexpressing Mcl-1, but not Eμ-myc lymphoma cells overexpressing Bcl-2. Treatment of human leukemic cell lines with compound 21 resulted in cell death through activation of caspase-3 and induction of apoptosis.
Figures

 , CH3CN, 80 °C, 15
h; (e) BBr3, CH2Cl2, 0 °C to
rt, 1 h, or BBr3, CH2Cl2, 0 °C
to rt, 1 h, quench with MeOH at 0 °C; (g) phenyl boronic acid,
Pd(PPh3)4, Na2CO3, THF/H2O, 60 °C, 2 h; (h) NH4OH, rt, 1 h; (i) NaN3, SiCl4, CH3CN, 80 °C, 15 h; (j)
LiOH, THF, rt, 1 h; (k) H3CCOCl, Et3N, 0 °C,
rt, 30 min.
, CH3CN, 80 °C, 15
h; (e) BBr3, CH2Cl2, 0 °C to
rt, 1 h, or BBr3, CH2Cl2, 0 °C
to rt, 1 h, quench with MeOH at 0 °C; (g) phenyl boronic acid,
Pd(PPh3)4, Na2CO3, THF/H2O, 60 °C, 2 h; (h) NH4OH, rt, 1 h; (i) NaN3, SiCl4, CH3CN, 80 °C, 15 h; (j)
LiOH, THF, rt, 1 h; (k) H3CCOCl, Et3N, 0 °C,
rt, 30 min.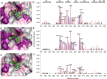

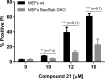

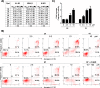
References
-
- Hanahan D.; Weinberg R. A. Hallmarks of cancer: the next generation. Cell 2011, 144, 646–674. - PubMed
-
- Hanahan D.; Weinberg R. A. The hallmarks of cancer. Cell 2000, 100, 57–70. - PubMed
-
- Fulda S.; Debatin K. M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. - PubMed
-
- Youle R. J.; Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Rev. Mol. Cell Biol. 2008, 9, 47–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials
Miscellaneous

