Chronic wasting disease agents in nonhuman primates
- PMID: 24751215
- PMCID: PMC4012792
- DOI: 10.3201/eid2005.130778
Chronic wasting disease agents in nonhuman primates
Abstract
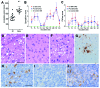
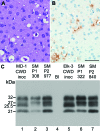
Chronic wasting disease is a prion disease of cervids. Assessment of its zoonotic potential is critical. To evaluate primate susceptibility, we tested monkeys from 2 genera. We found that 100% of intracerebrally inoculated and 92% of orally inoculated squirrel monkeys were susceptible, but cynomolgus macaques were not, suggesting possible low risk for humans.
Keywords: Chronic wasting disease; cynomolgus macaque; non-human primate; prion; species barrier; squirrel monkey; transmissible spongiform encephalopathy; transmission.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

